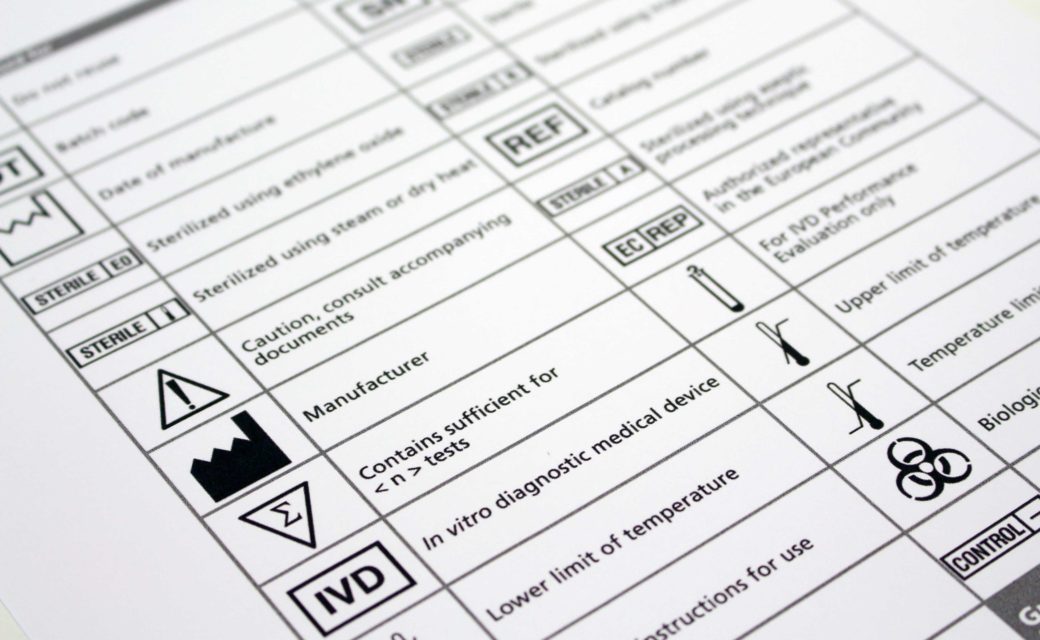
Medical Device Labeling Health Canada . There are also several additional requirements (for. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Health canada is the federal department responsible for helping the people of canada maintain and improve. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply.
from ambitiousmares.blogspot.com
Health canada is the federal department responsible for helping the people of canada maintain and improve. Medical devices are classified into one of 4 classes. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply.
34 Medical Device Label Symbols Labels Design Ideas 2020
Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. We are committed to ensuring that such requests. Class i represents the lowest risk and class iv represents the highest risk. There are also several additional requirements (for. Health canada is the federal department responsible for helping the people of canada maintain and improve. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply.
From mavink.com
Medical Device Labeling Symbols Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Class i represents the lowest risk and class iv represents the highest risk. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination with another. Medical Device Labeling Health Canada.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical devices are classified into one of 4 classes. There are also several additional requirements (for. Class i represents the lowest risk. Medical Device Labeling Health Canada.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labeling Health Canada We are committed to ensuring that such requests. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical devices are classified into one of 4 classes. Health canada is the federal department responsible for helping the people of canada maintain and improve. Class i represents the lowest. Medical Device Labeling Health Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Health canada is the federal department responsible for helping the people of canada maintain and improve. We are committed to ensuring that such requests. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended. Medical Device Labeling Health Canada.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Health canada is the federal department responsible for helping the people of canada maintain and improve. This allows us to adequately assess the safety, effectiveness or quality of a medical device. We are committed to ensuring that such requests. There are also several additional requirements (for. Active diagnostic device means an. Medical Device Labeling Health Canada.
From www.reedtech.com
Unique Device Identification (UDI) Lexis Nexis Reed Tech Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. There are also several additional requirements (for. Class i represents the lowest risk and class iv represents the highest risk.. Medical Device Labeling Health Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Class i represents the lowest risk and class iv represents the highest risk. Health. Medical Device Labeling Health Canada.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Class i represents the lowest risk and class iv represents the highest risk. There are also several additional requirements (for. Medical devices are classified into one of 4 classes. Health canada is the federal department responsible for helping the people of canada maintain and improve.. Medical Device Labeling Health Canada.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide Medical Device Labeling Health Canada Class i represents the lowest risk and class iv represents the highest risk. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. There are also several additional requirements (for. We are committed to ensuring that such requests. Health. Medical Device Labeling Health Canada.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. There are also several additional requirements (for. This allows us to adequately assess the safety, effectiveness or quality of a medical device. We are committed to ensuring that such. Medical Device Labeling Health Canada.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Health canada is the federal department responsible for helping the people of canada maintain and improve. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended. Medical Device Labeling Health Canada.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Device Labeling Health Canada Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical devices are classified into one of 4 classes. This allows us to adequately assess the safety, effectiveness or quality of a medical device. There are also several additional requirements (for. Health canada is the federal department responsible. Medical Device Labeling Health Canada.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device that, whether used alone or in combination. Medical Device Labeling Health Canada.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Medical Device Labeling Health Canada Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device that,. Medical Device Labeling Health Canada.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Health Canada There are also several additional requirements (for. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. We are committed to ensuring that such requests. Health canada is the federal department responsible for. Medical Device Labeling Health Canada.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labeling Health Canada We are committed to ensuring that such requests. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical devices are classified into one of 4 classes. Health canada is the federal department responsible for helping the people of canada maintain and improve. There are also several additional. Medical Device Labeling Health Canada.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Medical devices are classified into one of 4 classes. There are also several additional requirements (for. We are committed to ensuring that such requests. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended. Medical Device Labeling Health Canada.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical. Medical Device Labeling Health Canada.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Medical Device Labeling Health Canada Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. There are also several additional requirements (for. Medical devices are classified into one of 4 classes. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. This. Medical Device Labeling Health Canada.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Vascular Access Medical Device Labeling Health Canada There are also several additional requirements (for. Class i represents the lowest risk and class iv represents the highest risk. Health canada is the federal department responsible for helping the people of canada maintain and improve. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with. Medical Device Labeling Health Canada.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Health Canada We are committed to ensuring that such requests. Class i represents the lowest risk and class iv represents the highest risk. There are also several additional requirements (for. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that,. Medical Device Labeling Health Canada.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Medical Device Labeling Health Canada Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Health canada is the federal department responsible for helping the people of canada maintain and improve. Medical devices are classified into one of. Medical Device Labeling Health Canada.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. There are also several additional requirements (for. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Class i represents the lowest risk and. Medical Device Labeling Health Canada.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Class i represents the lowest risk and class iv represents the highest risk. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Medical devices are classified into one of 4 classes. There. Medical Device Labeling Health Canada.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labeling Health Canada There are also several additional requirements (for. Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. We are committed to ensuring that such requests. This allows us to adequately assess the safety, effectiveness or quality of a medical. Medical Device Labeling Health Canada.
From www.canada.ca
How Health Canada inspects medical device establishments About inspections Canada.ca Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. We are committed to ensuring that such requests. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Class i represents the lowest risk. Medical Device Labeling Health Canada.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. There are also several additional requirements (for. Health canada is the federal department responsible for. Medical Device Labeling Health Canada.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. This allows us to adequately assess the safety, effectiveness or quality of a medical device. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply.. Medical Device Labeling Health Canada.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Medical Device Labeling Health Canada Medical devices are classified into one of 4 classes. There are also several additional requirements (for. We are committed to ensuring that such requests. Class i represents the lowest risk and class iv represents the highest risk. Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device. Medical Device Labeling Health Canada.
From www.regdesk.co
Health Canada Guidance on Private Label Medical Devices RegDesk Medical Device Labeling Health Canada We are committed to ensuring that such requests. Health canada is the federal department responsible for helping the people of canada maintain and improve. This allows us to adequately assess the safety, effectiveness or quality of a medical device. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination. Medical Device Labeling Health Canada.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Labeling Health Canada Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. We are committed to ensuring that such requests. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of 4 classes. Class i represents the lowest risk. Medical Device Labeling Health Canada.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Class i represents the lowest risk and class iv represents the highest risk. We are committed to ensuring that such requests. Medical devices are classified into one of 4 classes. This allows us to adequately assess the safety, effectiveness or quality of a medical. Medical Device Labeling Health Canada.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Medical Device Labeling Health Canada This allows us to adequately assess the safety, effectiveness or quality of a medical device. We are committed to ensuring that such requests. There are also several additional requirements (for. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. Health canada is the federal department responsible for. Medical Device Labeling Health Canada.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Vascular Access Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. There are also several additional requirements (for. We are committed to ensuring that such requests. This allows us to adequately assess the. Medical Device Labeling Health Canada.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Medical Device Labeling Health Canada Health canada is the federal department responsible for helping the people of canada maintain and improve. Active diagnostic device means an active device that, whether used alone or in combination with another medical device, is intended to supply. This allows us to adequately assess the safety, effectiveness or quality of a medical device. Medical devices are classified into one of. Medical Device Labeling Health Canada.