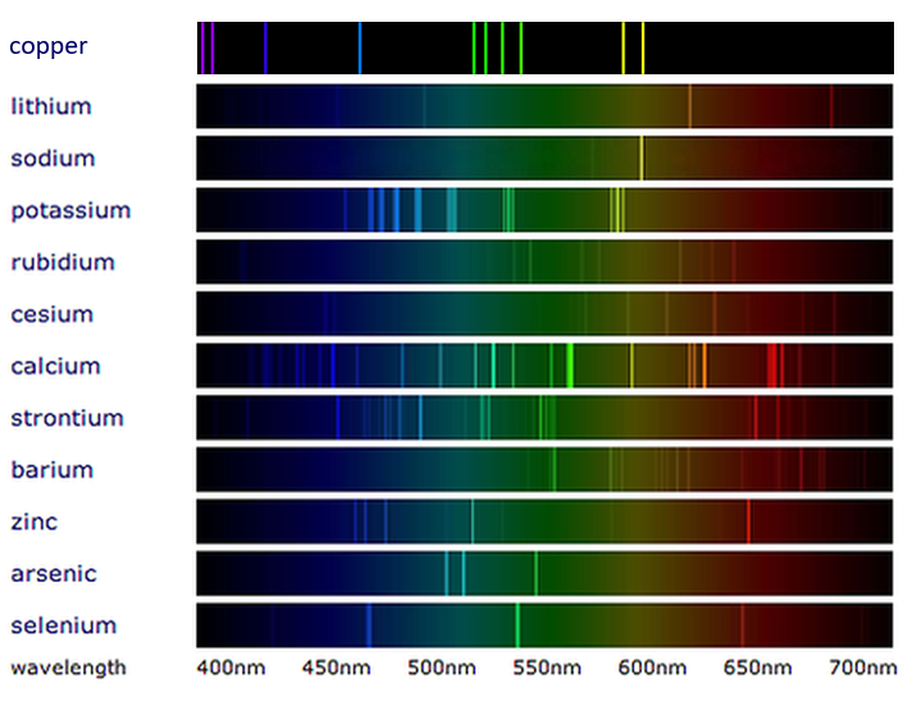
What Is Line Spectrum And Band Spectrum . In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. It may result from emission or absorption of light. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Band spectrum refers to continuous spectrum with bands, typical of. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to.
from www.mrpalermo.com
Band spectrum refers to continuous spectrum with bands, typical of. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. It may result from emission or absorption of light. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines.
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom
What Is Line Spectrum And Band Spectrum Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. Band spectrum refers to continuous spectrum with bands, typical of. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. It may result from emission or absorption of light. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to.. What Is Line Spectrum And Band Spectrum.
From phys.libretexts.org
2.3 The Spectrum Physics LibreTexts What Is Line Spectrum And Band Spectrum Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. Band spectrum refers to continuous spectrum with bands, typical of. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectra is the name given to groups of lines so closely spaced that each. What Is Line Spectrum And Band Spectrum.
From imagine.gsfc.nasa.gov
Spectra Introduction What Is Line Spectrum And Band Spectrum Band spectrum refers to continuous spectrum with bands, typical of. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A line spectrum appears as a series of. What Is Line Spectrum And Band Spectrum.
From www.difference.wiki
Line Spectrum vs. Band Spectrum What’s the Difference? What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Band spectrum refers to continuous spectrum with bands, typical of. Band spectra. What Is Line Spectrum And Band Spectrum.
From www.dreamstime.com
Spectrum with Each Band Usage Stock Illustration Illustration of spectrum What Is Line Spectrum And Band Spectrum The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectra is a phenomenon which occurs when excited atoms emit. What Is Line Spectrum And Band Spectrum.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum What Is Line Spectrum And Band Spectrum When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Band spectra is the name given to groups of lines so closely spaced that each group appears to be. What Is Line Spectrum And Band Spectrum.
From www.bench.com
Do You Know Your Frequency Bands? What Is Line Spectrum And Band Spectrum When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. It may result from emission or absorption of light. Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be seen. What Is Line Spectrum And Band Spectrum.
From pediaa.com
Difference Between Continuous Spectrum and Line Spectrum What Is Line Spectrum And Band Spectrum A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Band spectra is the name given to groups of lines so closely spaced that each group appears to be. What Is Line Spectrum And Band Spectrum.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room What Is Line Spectrum And Band Spectrum Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Band spectrum. What Is Line Spectrum And Band Spectrum.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b What Is Line Spectrum And Band Spectrum Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Line spectra is a phenomenon which occurs when. What Is Line Spectrum And Band Spectrum.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. The main difference between continuous spectrum and line spectrum is that line. What Is Line Spectrum And Band Spectrum.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and space science, Light What Is Line Spectrum And Band Spectrum Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather. What Is Line Spectrum And Band Spectrum.
From chemistrybook2011.blogspot.com
knowledge sea ATOMIC SPECTRUM What Is Line Spectrum And Band Spectrum Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band. What Is Line Spectrum And Band Spectrum.
From www.researchgate.net
Spectrum Bands and their wavelengths [19] Download Scientific Diagram What Is Line Spectrum And Band Spectrum Band spectrum refers to continuous spectrum with bands, typical of. It may result from emission or absorption of light. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. A spectral line is a weaker or stronger region in an otherwise uniform and continuous. What Is Line Spectrum And Band Spectrum.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download ID1755366 What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. When the emitted light is passed through a. What Is Line Spectrum And Band Spectrum.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom What Is Line Spectrum And Band Spectrum It may result from emission or absorption of light. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated. What Is Line Spectrum And Band Spectrum.
From infinitylearn.com
Line spectrum contains information about What Is Line Spectrum And Band Spectrum Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum,. What Is Line Spectrum And Band Spectrum.
From mynewblogsteetburt258.blogspot.com
WHAT IS SPECTRUM ? / EXPLAIN ITS FOLLOWING BANDS What Is Line Spectrum And Band Spectrum Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectrum refers to continuous spectrum with bands, typical of. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. When the emitted light is passed through a prism, only a few narrow lines of. What Is Line Spectrum And Band Spectrum.
From www.askdifference.com
Line Spectrum vs. Band Spectrum — What’s the Difference? What Is Line Spectrum And Band Spectrum A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Band. What Is Line Spectrum And Band Spectrum.
From www.youtube.com
What is band spectra YouTube What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. When the emitted light is passed through a prism, only a. What Is Line Spectrum And Band Spectrum.
From mavink.com
Spectrum Frequency Bands What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. The main difference between continuous spectrum and line spectrum is that line spectra can be seen. What Is Line Spectrum And Band Spectrum.
From users.highland.edu
Atomic Spectra and Models of the Atom What Is Line Spectrum And Band Spectrum It may result from emission or absorption of light. Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines, with huge. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as. What Is Line Spectrum And Band Spectrum.
From wisc.pb.unizin.org
Chapter 1 Light and Matter Chemistry 109 What Is Line Spectrum And Band Spectrum Band spectrum refers to continuous spectrum with bands, typical of. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Line spectra is a phenomenon which occurs when. What Is Line Spectrum And Band Spectrum.
From ar.inspiredpencil.com
Example Of Absorption Spectrum What Is Line Spectrum And Band Spectrum A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a. What Is Line Spectrum And Band Spectrum.
From www.britannica.com
HF frequency band Britannica What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. It may result from emission or absorption of light. Band spectrum refers to continuous spectrum with bands, typical of. The main difference between continuous spectrum and line spectrum is that line spectra can be. What Is Line Spectrum And Band Spectrum.
From www.slideserve.com
PPT Electron Arrangement PowerPoint Presentation ID1598428 What Is Line Spectrum And Band Spectrum A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. Band spectrum refers to continuous spectrum with bands, typical. What Is Line Spectrum And Band Spectrum.
From study.com
Continuous Spectrum Definition, Types & Examples Lesson What Is Line Spectrum And Band Spectrum It may result from emission or absorption of light. A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Band spectra is the name given to groups of lines. What Is Line Spectrum And Band Spectrum.
From flexautomotive.net
EMC FLEX BLOG Spectrum What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. It may result from emission or absorption of light. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectrum. What Is Line Spectrum And Band Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts What Is Line Spectrum And Band Spectrum Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. It may result from emission or absorption of light. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectra is a phenomenon which. What Is Line Spectrum And Band Spectrum.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum. YouTube What Is Line Spectrum And Band Spectrum In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to. It may result from emission or absorption of light. Band spectrum refers to continuous spectrum with. What Is Line Spectrum And Band Spectrum.
From www.researchgate.net
The spectrum, different wavelengths and regions, bands'... Download Scientific What Is Line Spectrum And Band Spectrum A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Band spectrum refers to continuous spectrum with bands, typical of. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. Line spectrum refers to discrete set of frequencies emitted. What Is Line Spectrum And Band Spectrum.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom What Is Line Spectrum And Band Spectrum A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths. What Is Line Spectrum And Band Spectrum.
From www.youtube.com
What is the Difference Between Continuous Spectrum and Line Spectrum Atomic Physics YouTube What Is Line Spectrum And Band Spectrum A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by. What Is Line Spectrum And Band Spectrum.
From www.slideserve.com
PPT Key Terms Chapter 7 PowerPoint Presentation, free download ID2979424 What Is Line Spectrum And Band Spectrum Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. What Is Line Spectrum And Band Spectrum.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption spectra show spectral lines What Is Line Spectrum And Band Spectrum Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. It may result from emission or absorption of light. Line spectra is a phenomenon which occurs when excited. What Is Line Spectrum And Band Spectrum.