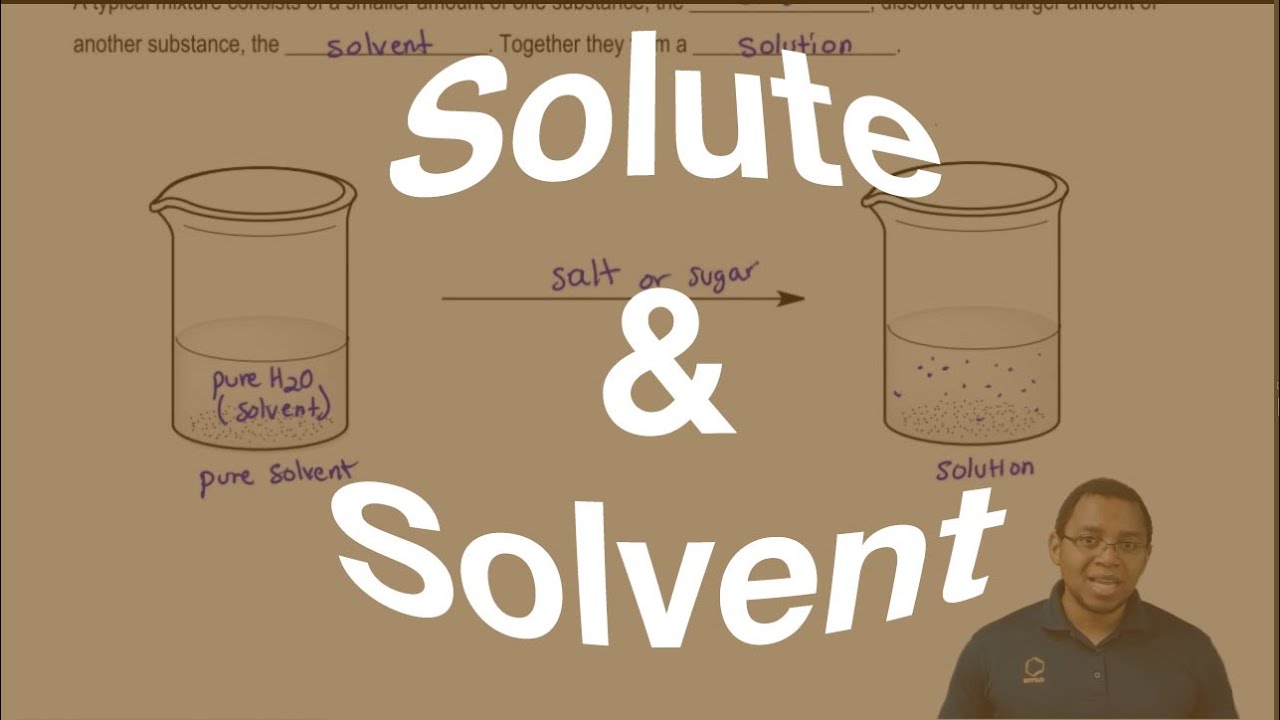
Is Liquid Detergent Solute Or Solvent . The solvent is the solution’s. the cleaning action of soaps and detergents can be explained in terms of the structures of the molecules involved. each minor component of a solution (and there may be more than one) is called the solute. soaps tend to be more solid, while detergents are usually in liquid form. A solvent is usually a liquid. the liquid is called the solvent. The solutes have two things in common. The most familiar solutions are aqueous. the solvent is the substance that dissolves the solute and the component of a chemical solution present in the. H 2 o and ccl 4. A solution can also be called a. a solution is made by dissolving 1.00 g of sucrose, c 12 h 22 o 11, in 100.0 g of liquid water. solvents are typically liquids, although they can also be solids or gases. The solvent breaks the larger solute particle into smaller particles, which are then dispersed throughout the solution. the solute is the substance that is being dissolved, while the solvent is the dissolving medium.
from hit.skku.edu
The solvent is the solution’s. They are both solids, and they. They do very similar things, and in household use. we call the major component the solvent and the minor component(s) the solute(s). solvents can be gases (such as nitrogen in the atmosphere), liquids (the most common liquid solvent is water), or. a chemical that is present in a solution can be classified as either a solute or a solvent. the solvent in detergents is typically water, while the solute is a mixture of cleaning agents, surfactants, enzymes,. I 2 and kmno 4. A solution can also be called a. In most of the solutions.
Solute Solvent Key Differences, Pros Cons, Examples, 43 OFF
Is Liquid Detergent Solute Or Solvent They are both solids, and they. a chemical that is present in a solution can be classified as either a solute or a solvent. Organic solvents contain carbon, whereas inorganic solvents are without carbon. Water is a solvent for polar molecules, and the. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. Solutions can be formed with many different types. They are both solids, and they. The solvent breaks the larger solute particle into smaller particles, which are then dispersed throughout the solution. solvents are typically liquids, although they can also be solids or gases. A solution can also be called a. the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. the basic difference between solute and solvent is that the former dissolves and the later is a dissolving medium. Solvent has a low boiling point and evaporates easily. it’s the liquid that the solute is dissolved in. The solutes have two things in common. in chemistry, a solute is the substance dissolved in a solvent or the part of a chemical solution present in the smaller amount.
From topseller.en.made-in-china.com
New Formula Detergent Powder Eco Friendly Detergent Powder Detergent Is Liquid Detergent Solute Or Solvent The solvent is the solution’s. H 2 o and ccl 4. The substance that has been dissolved is called the solute. the solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solutions can be formed with many different types. the liquid is called the solvent. a solution is a homogeneous mixture. Is Liquid Detergent Solute Or Solvent.
From ubicaciondepersonas.cdmx.gob.mx
Tide Liquid Brilliant Whites Liquid Detergent 1kg ubicaciondepersonas Is Liquid Detergent Solute Or Solvent the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. The solutes have two things in common. I 2 and kmno 4. A solution is a homogeneous mixture. The dispersed step of a solution is known as the solute. solvents are usually liquids, though they can be other forms of matter. They do very similar. Is Liquid Detergent Solute Or Solvent.
From www.indiamart.com
Lemon Blue Liquid Detergent Front Load, Packaging Type Can at Rs 1099 Is Liquid Detergent Solute Or Solvent The solute does not separate by filtration. the liquid is called the solvent. The solvent is the solution’s. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. The dispersed step of a solution is known as the solute. One of the most common solvents in the world is. Is Liquid Detergent Solute Or Solvent.
From hit.skku.edu
Solute Solvent Key Differences, Pros Cons, Examples, 43 OFF Is Liquid Detergent Solute Or Solvent a chemical that is present in a solution can be classified as either a solute or a solvent. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. The most familiar solutions are aqueous. the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. The. Is Liquid Detergent Solute Or Solvent.
From nashfix.us
Powder Detergent Vs Liquid Detergent NashFix Is Liquid Detergent Solute Or Solvent a chemical that is present in a solution can be classified as either a solute or a solvent. They do very similar things, and in household use. Solvent has a low boiling point and evaporates easily. The solvent is the solution’s. soaps tend to be more solid, while detergents are usually in liquid form. a solvent is. Is Liquid Detergent Solute Or Solvent.
From www.indiamart.com
Egret Matic Eco Friendly Liquid Detergent at Rs 550/piece whirlpool Is Liquid Detergent Solute Or Solvent In most of the solutions. When one substance dissolves into another, a solution is formed. The solute does not separate by filtration. One of the most common solvents in the world is water, and it is capable of dissolving. The dispersed step of a solution is known as the solute. The solvent is the solution’s. a solution is a. Is Liquid Detergent Solute Or Solvent.
From exockowmk.blob.core.windows.net
Best Laundry Detergent For Dingy Clothes at Hong Barnes blog Is Liquid Detergent Solute Or Solvent The solvent is the solution’s. They are both solids, and they. a solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. The dispersed step of a solution is known as the solute. A solution can also be. Is Liquid Detergent Solute Or Solvent.
From www.amazon.in
IFB ESSENTIALS Liquid Detergent (PACK OF 3) Amazon.in Health Is Liquid Detergent Solute Or Solvent The substance that has been dissolved is called the solute. solvents are typically liquids, although they can also be solids or gases. the basic difference between solute and solvent is that the former dissolves and the later is a dissolving medium. They are both solids, and they. each minor component of a solution (and there may be. Is Liquid Detergent Solute Or Solvent.
From www.sofw.com
Plantbased and Ecofriendly Liquid Laundry Detergent SOFW Verlag Is Liquid Detergent Solute Or Solvent I 2 and kmno 4. A solution is a homogeneous mixture. solvents are typically liquids, although they can also be solids or gases. in chemistry, a solute is the substance dissolved in a solvent or the part of a chemical solution present in the smaller amount. the solute is the substance that is being dissolved, while the. Is Liquid Detergent Solute Or Solvent.
From aradbranding.com
Sunlight Liquid Detergent Price in Kolkata Arad Branding Is Liquid Detergent Solute Or Solvent They do very similar things, and in household use. it’s the liquid that the solute is dissolved in. The most familiar solutions are aqueous. The solutes have two things in common. solvents can be gases (such as nitrogen in the atmosphere), liquids (the most common liquid solvent is water), or. A solution can also be called a. . Is Liquid Detergent Solute Or Solvent.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Is Liquid Detergent Solute Or Solvent the cleaning action of soaps and detergents can be explained in terms of the structures of the molecules involved. The most familiar solutions are aqueous. They do very similar things, and in household use. each minor component of a solution (and there may be more than one) is called the solute. A solution can also be called a.. Is Liquid Detergent Solute Or Solvent.
From ecolink.com
The Process of LiquidLiquid Extraction EcoLink Inc. Is Liquid Detergent Solute Or Solvent in chemistry, a solute is the substance dissolved in a solvent or the part of a chemical solution present in the smaller amount. Solvent has a low boiling point and evaporates easily. They do very similar things, and in household use. it’s the liquid that the solute is dissolved in. a solution is made by dissolving 1.00. Is Liquid Detergent Solute Or Solvent.
From www.lazada.com.ph
DIY Liquid Detergent Kit 17 Liters Yield Lazada PH Is Liquid Detergent Solute Or Solvent Solutions can be formed with many different types. a chemical that is present in a solution can be classified as either a solute or a solvent. soaps tend to be more solid, while detergents are usually in liquid form. A solution is a homogeneous mixture. The solvent is the solution’s. we call the major component the solvent. Is Liquid Detergent Solute Or Solvent.
From www.indiamart.com
5 Litre Liquid Detergent, For Cleaning For Is Liquid Detergent Solute Or Solvent I 2 and kmno 4. A solvent is usually a liquid. the basic difference between solute and solvent is that the former dissolves and the later is a dissolving medium. the solvent is the substance that dissolves the solute and the component of a chemical solution present in the. the solute is almost uniformly distributed in the. Is Liquid Detergent Solute Or Solvent.
From bettergoods.org
The Best NonToxic Laundry Detergent (40+ Products Analyzed) Is Liquid Detergent Solute Or Solvent The solutes have two things in common. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. A solvent is usually a liquid. A solution can also be called a. a chemical that is present in a solution can be classified as either a solute or a solvent. The. Is Liquid Detergent Solute Or Solvent.
From www.emsdiasum.com
Alkaline Liquid Detergent Is Liquid Detergent Solute Or Solvent The solutes have two things in common. the solute is the substance that is being dissolved, while the solvent is the dissolving medium. a chemical that is present in a solution can be classified as either a solute or a solvent. Solutions can be formed with many different types. The substance that has been dissolved is called the. Is Liquid Detergent Solute Or Solvent.
From brainly.ph
Activity 2 What Am I?Directions. Tell whether the following Is Liquid Detergent Solute Or Solvent They are both solids, and they. in chemistry, a solute is the substance dissolved in a solvent or the part of a chemical solution present in the smaller amount. Organic solvents contain carbon, whereas inorganic solvents are without carbon. a solution is made by dissolving 1.00 g of sucrose, c 12 h 22 o 11, in 100.0 g. Is Liquid Detergent Solute Or Solvent.
From www.indiamart.com
Blue Eco Rinse Detergent Liquid, Packaging Type Can, Packaging Size Is Liquid Detergent Solute Or Solvent a solution is made by dissolving 1.00 g of sucrose, c 12 h 22 o 11, in 100.0 g of liquid water. One of the most common solvents in the world is water, and it is capable of dissolving. A solution can also be called a. In most of the solutions. in chemistry, a solute is the substance. Is Liquid Detergent Solute Or Solvent.
From www.shutterstock.com
Dissolving Solvents RoyaltyFree Images, Stock Photos & Pictures Is Liquid Detergent Solute Or Solvent One of the most common solvents in the world is water, and it is capable of dissolving. A solution can also be called a. solvents can be gases (such as nitrogen in the atmosphere), liquids (the most common liquid solvent is water), or. the cleaning action of soaps and detergents can be explained in terms of the structures. Is Liquid Detergent Solute Or Solvent.
From yeserchem.com
Mastering Laundry Detergent Liquid Formulation A Comprehensive Guide Is Liquid Detergent Solute Or Solvent A solution is a homogeneous mixture. it’s the liquid that the solute is dissolved in. the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. soaps tend to be more solid, while detergents are usually in liquid form. solvents are typically liquids, although they can also be solids or gases. the solute. Is Liquid Detergent Solute Or Solvent.
From quizy.co.id
Quizy Deterjen Cair Liquid Detergent Blue 5L Quizy Clean Solutions Is Liquid Detergent Solute Or Solvent A solution can also be called a. the solute is almost uniformly distributed in the solvent, making a homogeneous mixture. the solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solvent has a low boiling point and evaporates easily. the solvent is the substance that dissolves the solute and the component. Is Liquid Detergent Solute Or Solvent.
From www.seventhgeneration.com
Liquid Laundry Detergent Free & Clear Seventh Generation Is Liquid Detergent Solute Or Solvent The solvent is the solution’s. a solution is made by dissolving 1.00 g of sucrose, c 12 h 22 o 11, in 100.0 g of liquid water. A solution is a homogeneous mixture. a chemical that is present in a solution can be classified as either a solute or a solvent. a solvent is usually a liquid. Is Liquid Detergent Solute Or Solvent.
From intercare-homedelivery.ae
Noon Liquid Detergent Oud 3L + Liquid Detergent Kandora 1L Intercare Is Liquid Detergent Solute Or Solvent a solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Solutions can be formed with many different types. In most of the solutions. The solvent is the chemical. the solvent in detergents is typically water, while the solute is a mixture of cleaning agents, surfactants, enzymes,. the liquid is. Is Liquid Detergent Solute Or Solvent.
From www.tradeindia.com
Liquid Detergent 5lt Apparel at Best Price in Vijayawada Speed Home Is Liquid Detergent Solute Or Solvent the liquid is called the solvent. solvents can be gases (such as nitrogen in the atmosphere), liquids (the most common liquid solvent is water), or. a chemical that is present in a solution can be classified as either a solute or a solvent. Water is a solvent for polar molecules, and the. Solvent has a low boiling. Is Liquid Detergent Solute Or Solvent.
From www.youtube.com
Difference between solute and solvent YouTube Is Liquid Detergent Solute Or Solvent the solvent is the substance that dissolves the solute and the component of a chemical solution present in the. A solvent is usually a liquid. we call the major component the solvent and the minor component(s) the solute(s). The solutes have two things in common. a solution is a homogeneous mixture composed of two or more substances,. Is Liquid Detergent Solute Or Solvent.
From www.tradeindia.com
Detergent Liquid Chemical Name Sodium Dodecyl Benzene Sulphonate at Is Liquid Detergent Solute Or Solvent a solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. each minor component of a solution (and there may be more than one) is called the solute. solvents are typically liquids, although they can also be solids or gases. A solution is a homogeneous mixture. solvents are usually. Is Liquid Detergent Solute Or Solvent.
From www.indiamart.com
Liquid Laundry Detergent, 5 Kg at Rs 450/kg in Erode ID 2852731706473 Is Liquid Detergent Solute Or Solvent the solvent is the substance that dissolves the solute and the component of a chemical solution present in the. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. In most of the solutions. in chemistry, a solute is the substance dissolved in a solvent or the part. Is Liquid Detergent Solute Or Solvent.
From worldemand.com
Liquid Laundry Detergents That Are Not Is Liquid Detergent Solute Or Solvent a chemical that is present in a solution can be classified as either a solute or a solvent. The solute does not separate by filtration. The substance that has been dissolved is called the solute. A solution can also be called a. One of the most common solvents in the world is water, and it is capable of dissolving.. Is Liquid Detergent Solute Or Solvent.
From www.slideserve.com
PPT Water C 1 Flashcards pp. 4553 PowerPoint Presentation, free Is Liquid Detergent Solute Or Solvent a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. solvents can be gases (such as nitrogen in the atmosphere), liquids (the most common liquid solvent is water), or. I 2 and kmno 4. Organic solvents contain carbon, whereas inorganic solvents are without carbon. solvents are typically liquids,. Is Liquid Detergent Solute Or Solvent.
From omni.fairprice.com.sg
Breeze Liquid Detergent Refill Colour Care NTUC FairPrice Is Liquid Detergent Solute Or Solvent One of the most common solvents in the world is water, and it is capable of dissolving. They do very similar things, and in household use. In most of the solutions. I 2 and kmno 4. A solution can also be called a. A solution is a homogeneous mixture. in chemistry, a solute is the substance dissolved in a. Is Liquid Detergent Solute Or Solvent.
From www.tomsguide.com
Powder vs liquid detergent which is best for your laundry? Tom's Guide Is Liquid Detergent Solute Or Solvent A solvent is usually a liquid. The solvent is the chemical. the solvent is the substance that dissolves the solute and the component of a chemical solution present in the. A solution can also be called a. The solvent breaks the larger solute particle into smaller particles, which are then dispersed throughout the solution. One of the most common. Is Liquid Detergent Solute Or Solvent.
From www.trishulhomecare.com
Detergent Powder vs Liquid Detergent Which is Right for You? Is Liquid Detergent Solute Or Solvent I 2 and kmno 4. the basic difference between solute and solvent is that the former dissolves and the later is a dissolving medium. The solvent is the solution’s. A solution is a homogeneous mixture. soaps tend to be more solid, while detergents are usually in liquid form. in chemistry, a solute is the substance dissolved in. Is Liquid Detergent Solute Or Solvent.
From www.youtube.com
Solute solvent and solution YouTube Is Liquid Detergent Solute Or Solvent The solvent breaks the larger solute particle into smaller particles, which are then dispersed throughout the solution. in chemistry, a solute is the substance dissolved in a solvent or the part of a chemical solution present in the smaller amount. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance. Is Liquid Detergent Solute Or Solvent.
From medium.com
Understanding the Importance of Always Using Liquid Detergent by Is Liquid Detergent Solute Or Solvent They do very similar things, and in household use. solvents are typically liquids, although they can also be solids or gases. a solution is made by dissolving 1.00 g of sucrose, c 12 h 22 o 11, in 100.0 g of liquid water. The substance that has been dissolved is called the solute. In most of the solutions.. Is Liquid Detergent Solute Or Solvent.
From exodwvjdr.blob.core.windows.net
Examples Of Solvent Things at Kevin Brownlee blog Is Liquid Detergent Solute Or Solvent Organic solvents contain carbon, whereas inorganic solvents are without carbon. the cleaning action of soaps and detergents can be explained in terms of the structures of the molecules involved. They are both solids, and they. a solution is a homogeneous mixture composed of two or more substances, where the solute is the substance being. The dispersed step of. Is Liquid Detergent Solute Or Solvent.