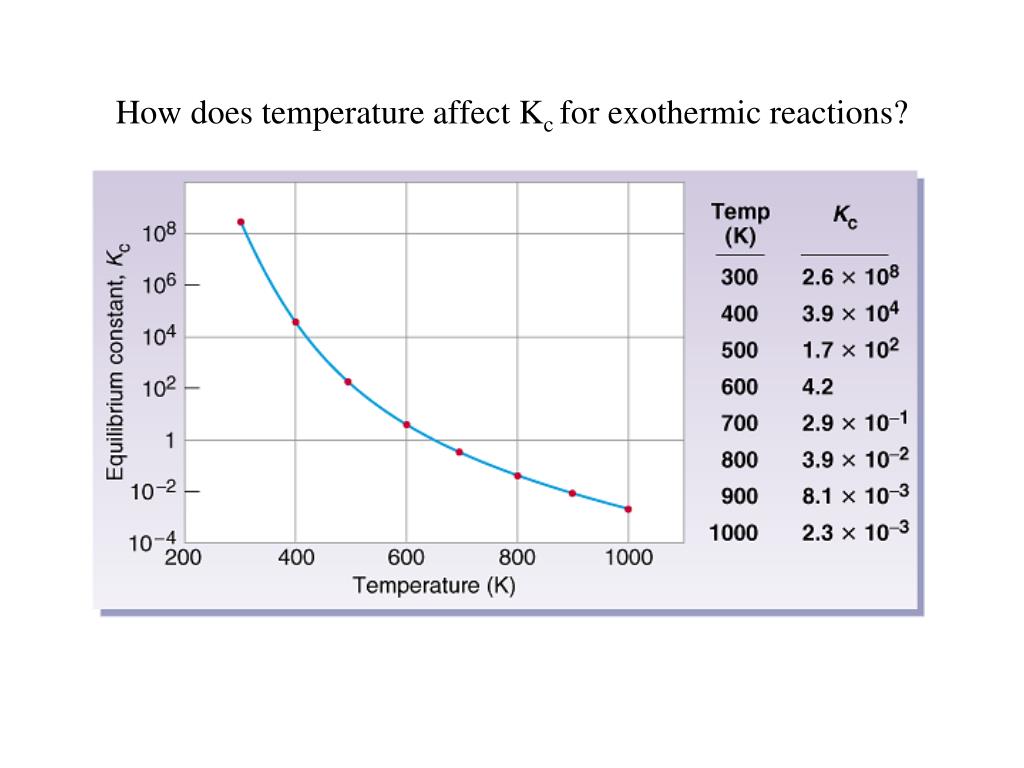
Does Kc Change With Temperature . Equilibrium constants are changed if you change the temperature of the system. Solubility increases as temperature increases. The units for k c can also be. Only temperature changes the value of k c. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. For an endothermic reaction such as: Changes in temperature change the equilibrium constant kc. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. Ks increases with temperature meaning dissolving is. The equilibrium constant is only affected by temperature. For exothermic and endothermic reactions, this added stress is a change in temperature. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. K c or k p are constant at constant temperature, but they vary as the temperature changes. The effect of temperature on kc and kp.
from www.slideserve.com
The equilibrium constant is only affected by temperature. The effect of temperature on kc and kp. For an endothermic reaction such as: It does not change if concentrations or pressures are varied, nor in the presence of catalysts. Changes in temperature change the equilibrium constant kc. Only temperature changes the value of k c. For exothermic and endothermic reactions, this added stress is a change in temperature. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. Ks increases with temperature meaning dissolving is. Equilibrium constants are changed if you change the temperature of the system.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Does Kc Change With Temperature Equilibrium constants are changed if you change the temperature of the system. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. Solubility increases as temperature increases. Changes in temperature change the equilibrium constant kc. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. Ks increases with temperature meaning dissolving is. Only temperature changes the value of k c. K c or k p are constant at constant temperature, but they vary as the temperature changes. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. For an endothermic reaction such as: The equilibrium constant is only affected by temperature. Equilibrium constants are changed if you change the temperature of the system. The units for k c can also be. For exothermic and endothermic reactions, this added stress is a change in temperature. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. The effect of temperature on kc and kp.
From learnah.org
3 Changing conditions on Kc and Kp Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. For an endothermic reaction such as: The equilibrium constant is only affected by temperature. Only temperature changes the value of k c. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. Equilibrium constants are changed if you change the temperature of the system. The effect. Does Kc Change With Temperature.
From mavink.com
Exothermic Reaction Temperature Graph Does Kc Change With Temperature The equilibrium constant is only affected by temperature. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. The units for k c can also be. For exothermic and endothermic reactions, this added stress is a change in temperature. Changes in temperature change. Does Kc Change With Temperature.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation ID3270711 Does Kc Change With Temperature It does not change if concentrations or pressures are varied, nor in the presence of catalysts. The equilibrium constant is only affected by temperature. For exothermic and endothermic reactions, this added stress is a change in temperature. Ks increases with temperature meaning dissolving is. But unlike a change in pressure, a change in temperature actually leads to a change in. Does Kc Change With Temperature.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. For an endothermic reaction such as: For exothermic and endothermic reactions, this added stress is a change in temperature. Ks increases with temperature meaning dissolving is. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. The effect of temperature on kc and kp.. Does Kc Change With Temperature.
From www.numerade.com
SOLVEDFor the watergas shift reaction CO(g)+H2 O(g) ⇌CO2(g)+H2(g), ΔH Does Kc Change With Temperature Ks increases with temperature meaning dissolving is. The effect of temperature on kc and kp. Equilibrium constants are changed if you change the temperature of the system. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. K c or k p are constant at constant temperature, but they vary as the. Does Kc Change With Temperature.
From musicbykatie.com
Does Temperature Increase Or Decrease Solubility In Endothermic Does Kc Change With Temperature Ks increases with temperature meaning dissolving is. Solubility increases as temperature increases. Equilibrium constants are changed if you change the temperature of the system. The equilibrium constant is only affected by temperature. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. The effect of temperature on kc. Does Kc Change With Temperature.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Does Kc Change With Temperature Solubility increases as temperature increases. The equilibrium constant is only affected by temperature. The effect of temperature on kc and kp. Changes in temperature change the equilibrium constant kc. The units for k c can also be. K c or k p are constant at constant temperature, but they vary as the temperature changes. Equilibrium constants are changed if you. Does Kc Change With Temperature.
From www.tec-science.com
Why does the temperature remain constant during a change of state Does Kc Change With Temperature It does not change if concentrations or pressures are varied, nor in the presence of catalysts. For exothermic and endothermic reactions, this added stress is a change in temperature. For an endothermic reaction such as: Solubility increases as temperature increases. Changes in temperature change the equilibrium constant kc. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\). Does Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Kc Change With Temperature Equilibrium constants are changed if you change the temperature of the system. Solubility increases as temperature increases. Only temperature changes the value of k c. Changes in temperature change the equilibrium constant kc. K c or k p are constant at constant temperature, but they vary as the temperature changes. It does not change if concentrations or pressures are varied,. Does Kc Change With Temperature.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does Kc Change With Temperature Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. For exothermic and endothermic reactions, this added stress is a change in temperature. For an endothermic reaction such as: Solubility increases as temperature increases. Changes in temperature change the equilibrium constant kc. But unlike a change in pressure, a change in temperature. Does Kc Change With Temperature.
From www.toppr.com
19. Consider the following equilibrium in a closed container N204(9) Al Does Kc Change With Temperature Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. The equilibrium constant is only affected by temperature. For exothermic and endothermic reactions, this added. Does Kc Change With Temperature.
From www.numerade.com
SOLVED predict the change in the equilibrium constant KC that would Does Kc Change With Temperature Ks increases with temperature meaning dissolving is. Changes in temperature change the equilibrium constant kc. Solubility increases as temperature increases. The effect of temperature on kc and kp. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. Changing the concentrations of the reactants and products doesn’t change. Does Kc Change With Temperature.
From tfvp.org
Why Is Kc Affected By Temperature Unveiling The Thermodynamic Mystery Does Kc Change With Temperature For exothermic and endothermic reactions, this added stress is a change in temperature. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in. Does Kc Change With Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Kc Change With Temperature The effect of temperature on kc and kp. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. K c or k p are constant at constant temperature, but they vary as the temperature changes. It does not change if concentrations or pressures. Does Kc Change With Temperature.
From www.youtube.com
Temperature Dependence of Equilibrium Constant YouTube Does Kc Change With Temperature Equilibrium constants are changed if you change the temperature of the system. The equilibrium constant is only affected by temperature. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. Ks increases with temperature meaning dissolving is. For exothermic and endothermic reactions, this. Does Kc Change With Temperature.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Does Kc Change With Temperature For exothermic and endothermic reactions, this added stress is a change in temperature. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. K c or k p are constant at constant temperature, but they vary as the temperature changes. Changing the concentrations of the reactants and products doesn’t change the value of k. Does Kc Change With Temperature.
From byjus.com
Kp and kc relationship below temperature 12.5k Does Kc Change With Temperature For an endothermic reaction such as: Solubility increases as temperature increases. Ks increases with temperature meaning dissolving is. Changes in temperature change the equilibrium constant kc. The units for k c can also be. Only temperature changes the value of k c. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. The equilibrium. Does Kc Change With Temperature.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2579442 Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. Equilibrium constants are changed if you change the temperature of the system. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. For exothermic and endothermic reactions, this added stress is a change in temperature. For an endothermic reaction such. Does Kc Change With Temperature.
From xo-b.com
In Which of the Following Reactions Will Kc Kp Does Kc Change With Temperature Equilibrium constants are changed if you change the temperature of the system. For exothermic and endothermic reactions, this added stress is a change in temperature. Changes in temperature change the equilibrium constant kc. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number.. Does Kc Change With Temperature.
From www.chegg.com
3. The effect of temperature on Kp is given by And Does Kc Change With Temperature An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. K c or k p are constant at constant temperature, but they vary as the temperature changes.. Does Kc Change With Temperature.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. The effect of temperature on kc and kp. The equilibrium constant is only affected by temperature. Equilibrium constants are changed if you change the temperature of the system. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. Ks increases with temperature meaning dissolving. Does Kc Change With Temperature.
From www.chegg.com
Solved Part B It the temperature is raised by 100 K, does Does Kc Change With Temperature Solubility increases as temperature increases. The effect of temperature on kc and kp. Equilibrium constants are changed if you change the temperature of the system. K c or k p are constant at constant temperature, but they vary as the temperature changes. For an endothermic reaction such as: Only temperature changes the value of k c. The equilibrium constant is. Does Kc Change With Temperature.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Does Kc Change With Temperature K c or k p are constant at constant temperature, but they vary as the temperature changes. For exothermic and endothermic reactions, this added stress is a change in temperature. Changes in temperature change the equilibrium constant kc. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!.. Does Kc Change With Temperature.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Does Kc Change With Temperature Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. The equilibrium constant is only affected by temperature. For an endothermic reaction such as: Solubility increases as temperature increases. An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the. Does Kc Change With Temperature.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. The equilibrium constant is only affected by temperature. The effect of temperature on kc and kp. Changing the concentrations of the reactants and products doesn’t change the value of k. Does Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Kc Change With Temperature The equilibrium constant is only affected by temperature. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. Equilibrium constants are changed if you change the temperature of the system. K c or k p are constant at constant temperature, but they vary as the temperature changes. Ks. Does Kc Change With Temperature.
From byjus.com
6. Why the value of kp and kc depends on the temperature only but not Does Kc Change With Temperature The units for k c can also be. For exothermic and endothermic reactions, this added stress is a change in temperature. Changes in temperature change the equilibrium constant kc. For an endothermic reaction such as: But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. Ks increases with. Does Kc Change With Temperature.
From socratic.org
How do I relate equilibrium constants to temperature change to find the Does Kc Change With Temperature Changes in temperature change the equilibrium constant kc. The units for k c can also be. The effect of temperature on kc and kp. Ks increases with temperature meaning dissolving is. Equilibrium constants are changed if you change the temperature of the system. K c or k p are constant at constant temperature, but they vary as the temperature changes.. Does Kc Change With Temperature.
From byjus.com
Explain with a graph, how does density of water varies with temperature Does Kc Change With Temperature Equilibrium constants are changed if you change the temperature of the system. Only temperature changes the value of k c. The effect of temperature on kc and kp. Changes in temperature change the equilibrium constant kc. It does not change if concentrations or pressures are varied, nor in the presence of catalysts. An equilibrium constant calculated from partial pressures (\(k_p\)). Does Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Kc Change With Temperature An equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and the change in the number. K c or k p are constant at constant temperature, but they vary as the temperature changes. But unlike a change in pressure, a change in temperature actually leads to a change in. Does Kc Change With Temperature.
From www.slideserve.com
PPT Equilibrium and Stability Constants PowerPoint Presentation, free Does Kc Change With Temperature But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant!. Changes in temperature change the equilibrium constant kc. Changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. Equilibrium constants are changed if you change the temperature of the system.. Does Kc Change With Temperature.
From www.caee.utexas.edu
Density vs. Temperature Does Kc Change With Temperature It does not change if concentrations or pressures are varied, nor in the presence of catalysts. The effect of temperature on kc and kp. K c or k p are constant at constant temperature, but they vary as the temperature changes. But unlike a change in pressure, a change in temperature actually leads to a change in the value of. Does Kc Change With Temperature.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation Does Kc Change With Temperature The units for k c can also be. K c or k p are constant at constant temperature, but they vary as the temperature changes. The effect of temperature on kc and kp. For exothermic and endothermic reactions, this added stress is a change in temperature. Equilibrium constants are changed if you change the temperature of the system. Only temperature. Does Kc Change With Temperature.
From www.youtube.com
19 Only temperature affects Kc YouTube Does Kc Change With Temperature K c or k p are constant at constant temperature, but they vary as the temperature changes. For exothermic and endothermic reactions, this added stress is a change in temperature. The units for k c can also be. Changes in temperature change the equilibrium constant kc. Solubility increases as temperature increases. Equilibrium constants are changed if you change the temperature. Does Kc Change With Temperature.
From www.youtube.com
Gibbs Energy change with temperature YouTube Does Kc Change With Temperature It does not change if concentrations or pressures are varied, nor in the presence of catalysts. Only temperature changes the value of k c. Equilibrium constants are changed if you change the temperature of the system. Changes in temperature change the equilibrium constant kc. For an endothermic reaction such as: But unlike a change in pressure, a change in temperature. Does Kc Change With Temperature.