Fuel Kinetic Energy . Calculate the kinetic energy of a gas molecule, given its temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. That is, they do not change the. Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature.
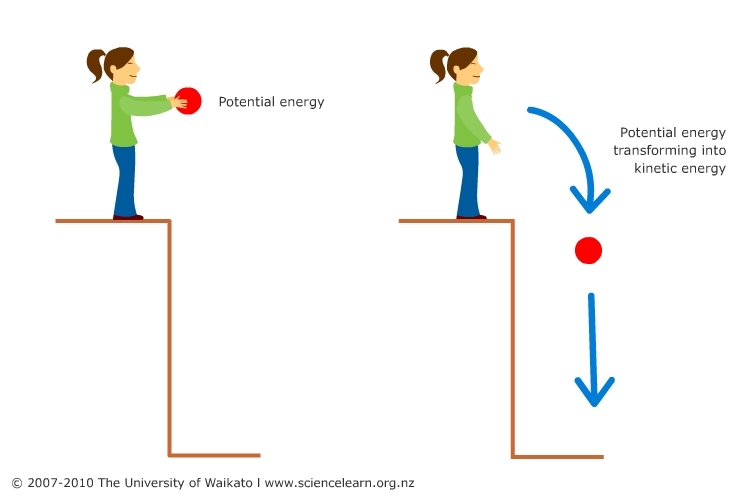
from www.sciencelearn.org.nz
According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Describe the relationship between the temperature of a gas and the. That is, they do not change the. Calculate the kinetic energy of a gas molecule, given its temperature.
Potential and energy — Science Learning Hub
Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Calculate the kinetic energy of a gas molecule, given its temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Describe the relationship between the temperature of a gas and the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. That is, they do not change the.
From www.learnatnoon.com
Difference between Potential and Energy Learn At Noon Fuel Kinetic Energy Calculate the kinetic energy of a gas molecule, given its temperature. Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are. Fuel Kinetic Energy.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs Fuel Kinetic Energy Calculate the kinetic energy of a gas molecule, given its temperature. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Describe the relationship between the temperature of a gas and the. That is, they do not change the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional. Fuel Kinetic Energy.
From www.1energysystems.com
Energy Understanding its Origins and Significance Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Calculate the kinetic energy of a gas molecule, given its temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; That is, they do not. Fuel Kinetic Energy.
From math.gallery.video
What is Energy & WorkEnergy Theorem in Physics? [18] Math Fuel Kinetic Energy Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. That is, they do not change the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly. Fuel Kinetic Energy.
From quizlet.com
Thermal, and Potential Energy Diagram Quizlet Fuel Kinetic Energy Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. That is, they do not change the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. Fuel Kinetic Energy.
From sciencenotes.org
What Is Energy? Energy Examples Fuel Kinetic Energy Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Describe the relationship between the temperature of a gas and the. Chemical. Fuel Kinetic Energy.
From www.windpowerengineering.com
Hawaiian Electric launches fourhour energy storage system Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas. Fuel Kinetic Energy.
From eduinput.com
EnergyDefinition,Types,And Work Energy Principle in Term of Fuel Kinetic Energy Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Calculate the kinetic energy of a gas molecule, given its temperature. That is, they do not change the. Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an. Fuel Kinetic Energy.
From www.dreamstime.com
and Potential Energy Explanation Labeled Vector Illustration Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Kinetic energy related to the forces acting on a. Fuel Kinetic Energy.
From innovationdiscoveries.space
Energy Recovery System (KERS) Fuel Kinetic Energy That is, they do not change the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Describe the relationship between the temperature of a gas and the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic. Fuel Kinetic Energy.
From www.slideserve.com
PPT Lecture 4 . Fuel Cell Reaction PowerPoint Presentation Fuel Kinetic Energy That is, they do not change the. Describe the relationship between the temperature of a gas and the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of. Fuel Kinetic Energy.
From www.out-class.org
Difference Between Potential and Energy OutClass Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Calculate the kinetic energy of a gas molecule, given its temperature. According to the kinetic molecular. Fuel Kinetic Energy.
From www.thoughtco.com
10 Types of Energy and Examples Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. That is, they do not change the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Calculate. Fuel Kinetic Energy.
From mungfali.com
10 Examples Of Energy Fuel Kinetic Energy According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Describe the relationship between the temperature of a gas and the. Calculate the kinetic energy of a gas molecule, given its temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy. Fuel Kinetic Energy.
From www.etutorworld.com
and Potential Energy Definitions, Key Difference, Examples and Fuel Kinetic Energy Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Calculate the kinetic energy of a gas molecule, given its temperature. Chemical kinetic reaction mechanisms for modern combustion systems are. Fuel Kinetic Energy.
From www.buzzle.com
The 13 Types of Energy and Their Varied Applications and Functions Fuel Kinetic Energy That is, they do not change the. Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic. Fuel Kinetic Energy.
From in.pinterest.com
Energy vs. Potential Energy What's The Difference (With Table Fuel Kinetic Energy Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly. Fuel Kinetic Energy.
From physicscalculations.com
How to Calculate Energy Without Velocity Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Calculate the kinetic energy of a gas molecule, given its temperature. According to the kinetic molecular theory, the average kinetic energy of. Fuel Kinetic Energy.
From polarpedia.eu
energy Polarpedia Fuel Kinetic Energy Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Describe the relationship between the temperature of a gas and the. Calculate the kinetic energy of a gas molecule, given its temperature. Gas molecules collide with one another and with the walls of. Fuel Kinetic Energy.
From eduinput.com
Difference between Energy and Potential Energy Fuel Kinetic Energy Calculate the kinetic energy of a gas molecule, given its temperature. Describe the relationship between the temperature of a gas and the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Kinetic energy related to the forces acting on a body and was referred to as “the energy. Fuel Kinetic Energy.
From www.examples.com
Energy 20+ Examples, Definition, Formula, Types Fuel Kinetic Energy Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Calculate the kinetic energy of a. Fuel Kinetic Energy.
From www.pinterest.cl
The 2 types and 9 forms of Energy and Potential Learn Fuel Kinetic Energy According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. That is, they do not change the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly. Fuel Kinetic Energy.
From www.careerpower.in
Energy Definition, Example and Derivation Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. That is, they do not change the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is. Fuel Kinetic Energy.
From www.youtube.com
and Potential Energy Simplified YouTube Fuel Kinetic Energy Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; That is, they do not change the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Calculate the kinetic energy of a gas molecule, given its temperature. Describe the relationship between the temperature of a gas. Fuel Kinetic Energy.
From www.teachoo.com
Energy Definition, Formula, Examples Teachoo Fuel Kinetic Energy Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Calculate the kinetic energy of a gas molecule, given its temperature. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Describe the relationship between the temperature of a gas and the. Kinetic energy related to the. Fuel Kinetic Energy.
From enjoy-teaching.com
9 Forms of Energy and Examples for Kids Fuel Kinetic Energy Calculate the kinetic energy of a gas molecule, given its temperature. That is, they do not change the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is. Fuel Kinetic Energy.
From www.gcse.co.uk
and Potential Energy Stores Revision Notes • GCSE Physics Fuel Kinetic Energy That is, they do not change the. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Calculate the kinetic energy of a gas molecule, given its temperature. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Describe the relationship between the temperature of. Fuel Kinetic Energy.
From www.vecteezy.com
Potential and energy diagram. 27798551 Vector Art at Vecteezy Fuel Kinetic Energy Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and.. Fuel Kinetic Energy.
From owlcation.com
How to Understand Energy, Momentum and Work Done Owlcation Fuel Kinetic Energy Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. That is, they do not change the. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly. Fuel Kinetic Energy.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID5128069 Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. That is, they do not change the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas. Fuel Kinetic Energy.
From www.gktoday.in
What are eFuels? GKToday Fuel Kinetic Energy Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Calculate the kinetic energy of a gas molecule, given its temperature. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. Chemical. Fuel Kinetic Energy.
From www.sciencelearn.org.nz
Potential and energy — Science Learning Hub Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Calculate the kinetic energy of a gas molecule, given its temperature. That is, they do not change the. Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and. Kinetic energy related to the forces acting on a body and was referred to as “the energy. Fuel Kinetic Energy.
From www.yourdictionary.com
Energy Examples YourDictionary Fuel Kinetic Energy Describe the relationship between the temperature of a gas and the. Calculate the kinetic energy of a gas molecule, given its temperature. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature. That is, they do not change the. Gas molecules collide with one another and with the walls. Fuel Kinetic Energy.
From flyingcolorsscience.com
Graph How Speed and Mass Affect Energy Cars Examples Flying Fuel Kinetic Energy Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Describe the relationship between the temperature of a gas and the. Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. That is, they. Fuel Kinetic Energy.
From courses.lumenlearning.com
Energy and Metabolism OpenStax Biology 2e Fuel Kinetic Energy Kinetic energy related to the forces acting on a body and was referred to as “the energy of motion.” the kinetic energy of a particle is one. Gas molecules collide with one another and with the walls of the container, but these collisions are perfectly elastic; Chemical kinetic reaction mechanisms for modern combustion systems are described, including historical highlights and.. Fuel Kinetic Energy.