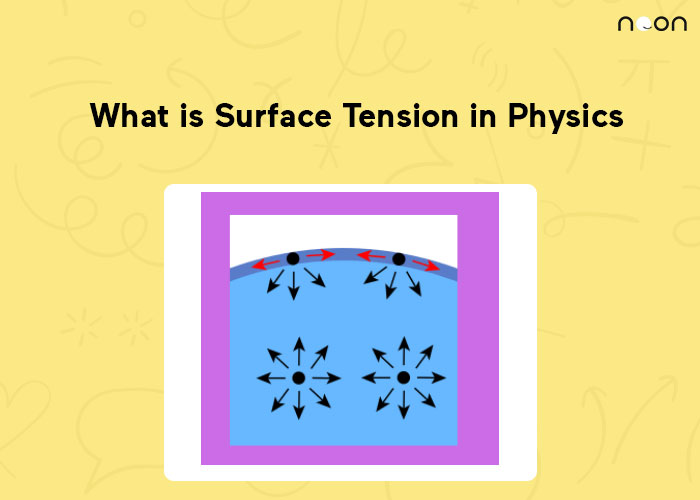
How Can Surface Tension Be Affected . In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. How does water purity affect surface tension? There is a common misconception for the source of the surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The surface tension will decrease if. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. For the analysis of the surface tension effects for. The presence of soluble particles in water may increase or decrease surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The molecules on the surface are.
from www.learnatnoon.com
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. The surface tension will decrease if. The molecules on the surface are. Surface tension results from a sharp change in the density between two adjoined phases or materials. For the analysis of the surface tension effects for. The presence of soluble particles in water may increase or decrease surface tension.
What is Surface Tension in Physics?
How Can Surface Tension Be Affected Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. How does water purity affect surface tension? Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. There is a common misconception for the source of the surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The surface tension will decrease if. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface are. Since these intermolecular forces vary depending on the nature of the. The presence of soluble particles in water may increase or decrease surface tension. For the analysis of the surface tension effects for.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Can Surface Tension Be Affected Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. How does water purity affect surface tension? Surface tension may be expressed, therefore, in units of energy per unit area (square. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download ID7090893 How Can Surface Tension Be Affected Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The presence of soluble particles in water may increase or decrease surface tension. How does water purity affect surface tension? Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. Surface tension results from a sharp change in. How Can Surface Tension Be Affected.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Consequences Chemistry Notes How Can Surface Tension Be Affected The molecules on the surface are. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. How does water purity affect surface tension? Surface tension is caused by liquid particle intermolecular forces such as the. How Can Surface Tension Be Affected.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Can Surface Tension Be Affected Surface tension may be expressed, therefore, in units of energy per unit area (square metres). There is a common misconception for the source of the surface tension. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The presence of soluble particles in water may increase or decrease surface tension. How does water purity. How Can Surface Tension Be Affected.
From slideplayer.com
Surface Tension The surface of any liquid behaves as if it was a stretched membrane. This How Can Surface Tension Be Affected Since these intermolecular forces vary depending on the nature of the. Surface tension results from a sharp change in the density between two adjoined phases or materials. How does water purity affect surface tension? Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. In the absence of other forces, the surface tension makes a. How Can Surface Tension Be Affected.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Can Surface Tension Be Affected Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The presence of soluble particles in water may increase or decrease surface tension. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. The molecules on the. How Can Surface Tension Be Affected.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Can Surface Tension Be Affected How does water purity affect surface tension? Surface tension results from a sharp change in the density between two adjoined phases or materials. There is a common misconception for the source of the surface tension. For the analysis of the surface tension effects for. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download ID1597821 How Can Surface Tension Be Affected The surface tension will decrease if. Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. How does water purity affect surface tension? Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The molecules on the surface. How Can Surface Tension Be Affected.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement How Can Surface Tension Be Affected Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The surface tension will decrease if. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does water purity affect surface tension? The molecules on the surface are. The presence of soluble particles in. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation ID2778612 How Can Surface Tension Be Affected The surface tension will decrease if. Surface tension results from a sharp change in the density between two adjoined phases or materials. Since these intermolecular forces vary depending on the nature of the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension is the energy, or work, required to increase the surface. How Can Surface Tension Be Affected.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Can Surface Tension Be Affected Surface tension results from a sharp change in the density between two adjoined phases or materials. For the analysis of the surface tension effects for. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. There is a common misconception for the source of the surface tension. Characteristics of a. How Can Surface Tension Be Affected.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Can Surface Tension Be Affected Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension will decrease if. Since these intermolecular forces vary depending on the nature of the. The molecules on the surface are. For the analysis of the surface tension effects for. Characteristics of a fluidic substance stay basically stable, but can be changed. How Can Surface Tension Be Affected.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Can Surface Tension Be Affected Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Water has a surface tension of 0.07275 joule per. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation ID2778612 How Can Surface Tension Be Affected Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Since these intermolecular forces vary depending on the nature of the. The surface tension will decrease if. Water has a surface tension of 0.07275 joule per square metre. How Can Surface Tension Be Affected.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Can Surface Tension Be Affected Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. For the analysis of the surface tension effects for. The presence of soluble particles in water. How Can Surface Tension Be Affected.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Illustration of science How Can Surface Tension Be Affected Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. Since these intermolecular forces vary depending on the. How Can Surface Tension Be Affected.
From testbook.com
Surface Tension & Capillarity with Application, Formula & Examples How Can Surface Tension Be Affected Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension results from a sharp change in the density between two adjoined phases or materials. Since these intermolecular forces vary depending on the nature of the. How does water purity affect surface tension? Characteristics of a fluidic substance stay basically stable, but can be. How Can Surface Tension Be Affected.
From byjus.com
Explain the surface tension phenomenon with examples. How Can Surface Tension Be Affected Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. The presence of soluble particles in water may increase or decrease surface tension. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize. How Can Surface Tension Be Affected.
From www.youtube.com
Surface Tension Fluid Mechanics Real Life Example Easy To Understand For Everyone YouTube How Can Surface Tension Be Affected Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does water purity affect surface tension? Surface tension may be expressed, therefore, in units of energy per unit area (square metres). The surface tension will decrease if. Surface tension results from a sharp change in the density between two. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free download ID1623857 How Can Surface Tension Be Affected The surface tension will decrease if. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension results from a sharp change in the density between two adjoined phases or materials. The presence of soluble particles in water may increase or decrease surface tension. The molecules on the surface are. There is a common. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Can Surface Tension Be Affected Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. There is a common misconception for the source of the surface tension. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Characteristics of a fluidic substance stay basically stable, but can be changed by. How Can Surface Tension Be Affected.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Capillary Action How Can Surface Tension Be Affected There is a common misconception for the source of the surface tension. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. How does water purity affect surface tension? The molecules on the surface are. Surface tension may be. How Can Surface Tension Be Affected.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Science, Science Lesson Plans How Can Surface Tension Be Affected The molecules on the surface are. Surface tension results from a sharp change in the density between two adjoined phases or materials. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The presence of soluble particles in water may increase or decrease surface tension. Surface tension is caused by liquid particle intermolecular forces. How Can Surface Tension Be Affected.
From www.learnatnoon.com
What is Surface Tension in Physics? How Can Surface Tension Be Affected The surface tension will decrease if. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). For the analysis of the surface tension effects for. The molecules on the surface are. The presence of soluble particles in water may increase or decrease surface tension. How does water purity affect surface tension? Surface tension is caused. How Can Surface Tension Be Affected.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences How Can Surface Tension Be Affected The presence of soluble particles in water may increase or decrease surface tension. How does water purity affect surface tension? In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. The molecules on the surface are. The surface tension will decrease if. Characteristics of a fluidic. How Can Surface Tension Be Affected.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Can Surface Tension Be Affected Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. How does water purity affect surface tension? Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275 joule per square metre. How Can Surface Tension Be Affected.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Can Surface Tension Be Affected Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. The surface tension will decrease if. How does water purity affect surface tension? Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. In. How Can Surface Tension Be Affected.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube How Can Surface Tension Be Affected The surface tension will decrease if. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Since these intermolecular forces vary depending on the nature of the. The molecules on the surface are. There is a common misconception for the source of the surface tension. For the analysis of the surface tension effects for.. How Can Surface Tension Be Affected.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces between water molecules How Can Surface Tension Be Affected Surface tension results from a sharp change in the density between two adjoined phases or materials. In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. Since these intermolecular forces vary depending on the nature of the. There is a common misconception for the source of. How Can Surface Tension Be Affected.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Consequences Chemistry Notes How Can Surface Tension Be Affected Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. Since these intermolecular forces vary depending on the nature of the. There is a common misconception for the source of the surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Surface tension results from a sharp. How Can Surface Tension Be Affected.
From learningmediawashermen.z14.web.core.windows.net
How To Explain Surface Tension To Kids How Can Surface Tension Be Affected Surface tension may be expressed, therefore, in units of energy per unit area (square metres). There is a common misconception for the source of the surface tension. Surface tension results from a sharp change in the density between two adjoined phases or materials. For the analysis of the surface tension effects for. The surface tension will decrease if. Surface tension. How Can Surface Tension Be Affected.
From www.youtube.com
How does Surface Tension work? YouTube How Can Surface Tension Be Affected In the absence of other forces, the surface tension makes a liquid drop spherical to minimize its surface area \(a\) at a fixed volume. There is a common misconception for the source of the surface tension. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area. How Can Surface Tension Be Affected.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Can Surface Tension Be Affected How does water purity affect surface tension? The surface tension will decrease if. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface are. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. For the analysis of the. How Can Surface Tension Be Affected.
From www.adda247.com
Surface Tension Definition, Formula, Examples How Can Surface Tension Be Affected Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. The presence of soluble particles in water may increase or decrease surface tension. The molecules on the surface are. Surface tension is caused by liquid particle intermolecular forces such as the van der waals force. Surface tension may be expressed, therefore, in units of energy. How Can Surface Tension Be Affected.
From www.youtube.com
Surface Tension of Water Explained YouTube How Can Surface Tension Be Affected The presence of soluble particles in water may increase or decrease surface tension. Characteristics of a fluidic substance stay basically stable, but can be changed by temperature variations, chemicals. Since these intermolecular forces vary depending on the nature of the. How does water purity affect surface tension? For the analysis of the surface tension effects for. Surface tension is caused. How Can Surface Tension Be Affected.