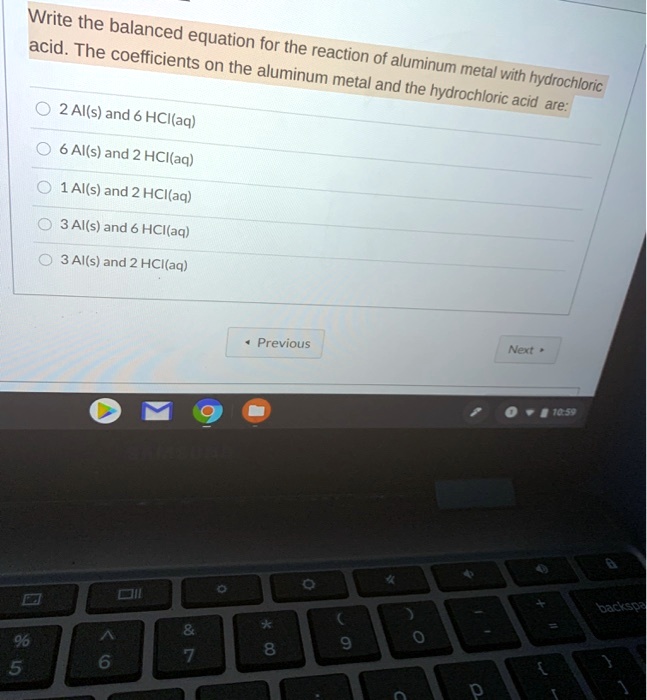
Aluminum Foil And Hydrochloric Acid Equation . The balanced equation of this reaction is as follows: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Aluminum acts as the reducing agent, giving up electrons: The aluminum comes from the aluminum foil,. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. In this problem, the salt produced will be aluminum chloride, or alcl3. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. 2al + 6hcl → 2alcl3 + 3h2 ↑. The reaction is irreversible as the products cannot be converted into reactants again. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen:
from www.numerade.com
Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: The balanced equation of this reaction is as follows: The reaction is irreversible as the products cannot be converted into reactants again. The aluminum comes from the aluminum foil,. 2al + 6hcl → 2alcl3 + 3h2 ↑. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this problem, the salt produced will be aluminum chloride, or alcl3.
SOLVED Write the balanced acid. The equation for the reaction
Aluminum Foil And Hydrochloric Acid Equation When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Aluminum acts as the reducing agent, giving up electrons: When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. 2al + 6hcl → 2alcl3 + 3h2 ↑. The balanced equation of this reaction is as follows: In this problem, the salt produced will be aluminum chloride, or alcl3. When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. The reaction is irreversible as the products cannot be converted into reactants again. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. The aluminum comes from the aluminum foil,. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen:
From www.numerade.com
SOLVED Chemical equations Write the following types of balanced Aluminum Foil And Hydrochloric Acid Equation In this problem, the salt produced will be aluminum chloride, or alcl3. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. 2al + 6hcl → 2alcl3 + 3h2 ↑. Aluminum acts. Aluminum Foil And Hydrochloric Acid Equation.
From www.coursehero.com
[Solved] 2. The reaction of aluminum with hydrochloric acid produces Aluminum Foil And Hydrochloric Acid Equation The aluminum comes from the aluminum foil,. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind. Aluminum Foil And Hydrochloric Acid Equation.
From www.slideserve.com
PPT Quiz PowerPoint Presentation, free download ID3201515 Aluminum Foil And Hydrochloric Acid Equation In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). The balanced equation of this reaction is as follows: Aluminum acts as the reducing agent, giving up electrons: In this problem, the salt produced will be aluminum chloride, or alcl3. The reaction is irreversible as the products cannot. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED When aluminum, Al, metal is dipped in an aqueous solution of Aluminum Foil And Hydrochloric Acid Equation In this problem, the salt produced will be aluminum chloride, or alcl3. The balanced equation of this reaction is as follows: 2al + 6hcl → 2alcl3 + 3h2 ↑. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. Explore the reactions. Aluminum Foil And Hydrochloric Acid Equation.
From fphoto.photoshelter.com
science chemistry exothermic reaction hydrochloric acid aluminum Aluminum Foil And Hydrochloric Acid Equation 2al + 6hcl → 2alcl3 + 3h2 ↑. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: The aluminum comes from the aluminum foil,. In this experiment, you will. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Amazing chemistry Aluminium foil reaction with hydrochloric acid Aluminum Foil And Hydrochloric Acid Equation The balanced equation of this reaction is as follows: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. Before proceeding with the experiment, read the. Aluminum Foil And Hydrochloric Acid Equation.
From blog.thepipingmart.com
What Happens when Aluminium Reacts with Hydrochloric acid Aluminum Foil And Hydrochloric Acid Equation Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. In this problem, the salt produced will be aluminum chloride, or alcl3. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. The reaction is irreversible as the products cannot be converted into reactants again.. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED From the following balanced equation, the mass of aluminum Aluminum Foil And Hydrochloric Acid Equation Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: In this problem, the salt produced will be aluminum chloride, or alcl3. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. The aluminum comes from the aluminum foil,. The reaction is irreversible as the products cannot be converted into reactants again. Aluminium metal will. Aluminum Foil And Hydrochloric Acid Equation.
From www.chegg.com
Solved Aluminum reacts with hydrochloric acid to give Aluminum Foil And Hydrochloric Acid Equation The balanced equation of this reaction is as follows: Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. The reaction. Aluminum Foil And Hydrochloric Acid Equation.
From www.nagwa.com
Question Video Explaining Why a Gas Is Produced When Aluminum Foil Is Aluminum Foil And Hydrochloric Acid Equation Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Before proceeding with the experiment, read the chemical formula below to. Aluminum Foil And Hydrochloric Acid Equation.
From www.chegg.com
Solved 5) Aluminum reacts with hydrochloric acid to produce Aluminum Foil And Hydrochloric Acid Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. 2al + 6hcl → 2alcl3 + 3h2 ↑. The balanced equation of this reaction is as follows: The aluminum comes from the aluminum foil,. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Find out which salt or ion,. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Hydrochloric acid vs Aluminium foilshortsviral status experiment Aluminum Foil And Hydrochloric Acid Equation Aluminum acts as the reducing agent, giving up electrons: In this problem, the salt produced will be aluminum chloride, or alcl3. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. 2al + 6hcl → 2alcl3 + 3h2 ↑. The balanced equation of this reaction is as follows: Explore the reactions between aluminium foil and. Aluminum Foil And Hydrochloric Acid Equation.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Reaction Of Aluminium Oxide With Hydrochloric Acid Aluminum Foil And Hydrochloric Acid Equation In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). The reaction is irreversible as the products cannot be converted into reactants again. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. The balanced equation of this reaction is as. Aluminum Foil And Hydrochloric Acid Equation.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Aluminum Foil And Hydrochloric Acid Equation When a metal reacts with an acid, a salt solution and hydrogen gas are produced. The balanced equation of this reaction is as follows: Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii). Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Hydrochloric Acid Reacting With Aluminum Foil YouTube Aluminum Foil And Hydrochloric Acid Equation 2al + 6hcl → 2alcl3 + 3h2 ↑. When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. The reaction is irreversible as the products cannot be converted into reactants again. The aluminum comes from the aluminum foil,. In this experiment, you. Aluminum Foil And Hydrochloric Acid Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Aluminum Foil And Hydrochloric Acid Equation In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Aluminum acts as the reducing agent, giving up electrons: Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. Cations of hydrochloric acid take these electrons and are reduced to molecular. Aluminum Foil And Hydrochloric Acid Equation.
From questions.kunduz.com
Aluminum reacts with hydrochloric acid f... Chemistry Aluminum Foil And Hydrochloric Acid Equation Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Aluminum acts as the reducing agent, giving up electrons: Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this experiment, you will prepare copper metal from. Aluminum Foil And Hydrochloric Acid Equation.
From allthingsaluminum.com
Reactivity Of Aluminum And Hydrochloric Acid All Things Aluminum Aluminum Foil And Hydrochloric Acid Equation In this problem, the salt produced will be aluminum chloride, or alcl3. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: 2al + 6hcl → 2alcl3 + 3h2 ↑. When a metal reacts with an acid, a salt solution and hydrogen gas are produced.. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Aluminium Foil & Hydrochloric acid (HCL) Reaction YouTube Aluminum Foil And Hydrochloric Acid Equation The balanced equation of this reaction is as follows: Aluminum acts as the reducing agent, giving up electrons: In this problem, the salt produced will be aluminum chloride, or alcl3. The aluminum comes from the aluminum foil,. The reaction is irreversible as the products cannot be converted into reactants again. Cations of hydrochloric acid take these electrons and are reduced. Aluminum Foil And Hydrochloric Acid Equation.
From www.coursehero.com
[Solved] When aluminum foil reacts with hydrochloric acid, it produces Aluminum Foil And Hydrochloric Acid Equation Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. The aluminum comes from the aluminum foil,. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: When a metal reacts with an acid, a salt solution and hydrogen gas are produced. The balanced equation of this reaction is as. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED Preface Aluminum foil was reacted will hydrochloric acid and Aluminum Foil And Hydrochloric Acid Equation When a metal reacts with an acid, a salt solution and hydrogen gas are produced. The balanced equation of this reaction is as follows: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Before proceeding with the experiment, read the chemical. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Aluminium(Al)+Hydrochloric acid(HCl).The chemical Equation between Aluminum Foil And Hydrochloric Acid Equation The balanced equation of this reaction is as follows: Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. 2al + 6hcl → 2alcl3 + 3h2 ↑. Aluminum acts as the reducing agent, giving up electrons: Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this problem, the salt. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Hydrochloric Acid and Aluminum Reaction YouTube Aluminum Foil And Hydrochloric Acid Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. The reaction is irreversible as the products cannot be converted. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
HOW TO MAKE BOMB?! HYDROCHLORIC ACID AND ALUMINIUM FOIL EXPERIMENT Aluminum Foil And Hydrochloric Acid Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). 2al + 6hcl → 2alcl3 + 3h2 ↑. The reaction is irreversible as the products cannot be converted into reactants again. Aluminum acts. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED What salt is formed in the reaction of aluminum with Aluminum Foil And Hydrochloric Acid Equation Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. Before proceeding. Aluminum Foil And Hydrochloric Acid Equation.
From mammothmemory.net
Aluminium reaction to hydrochloric acid is slow to start Aluminum Foil And Hydrochloric Acid Equation Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: 2al + 6hcl → 2alcl3 + 3h2 ↑. In this problem, the salt produced will be aluminum chloride, or alcl3. When a metal reacts with an acid, a salt solution and hydrogen gas are produced. The balanced equation of this reaction is as follows: Aluminium metal will. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
How to Balance Al + HCl = AlCl3 + H2 YouTube Aluminum Foil And Hydrochloric Acid Equation The reaction is irreversible as the products cannot be converted into reactants again. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: 2al + 6hcl → 2alcl3 + 3h2 ↑. Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. In this problem, the salt produced will be aluminum chloride, or alcl3. When aluminum. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Reaction of Aluminum and Hydrochloric acid (concentrated) YouTube Aluminum Foil And Hydrochloric Acid Equation 2al + 6hcl → 2alcl3 + 3h2 ↑. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). The balanced equation of this reaction is as follows: Aluminium metal will react. Aluminum Foil And Hydrochloric Acid Equation.
From www.youtube.com
Balancing Chemical Equations. Part 1 Aluminium and hydrochloric acid Aluminum Foil And Hydrochloric Acid Equation Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. In this problem, the salt produced will be aluminum chloride, or alcl3. When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of. Aluminum Foil And Hydrochloric Acid Equation.
From www.scribd.com
Aliminum Foil and Hydrochloric Acid (Effect of Concentration) PDF Aluminum Foil And Hydrochloric Acid Equation Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). The aluminum comes from the aluminum foil,. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. When. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED Write the balanced acid. The equation for the reaction Aluminum Foil And Hydrochloric Acid Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. In this problem, the salt produced will be aluminum chloride, or alcl3. The reaction is irreversible as the products cannot be converted into reactants again. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVEDaluminium metal strip is added in hydrochloric acid to produce Aluminum Foil And Hydrochloric Acid Equation In this problem, the salt produced will be aluminum chloride, or alcl3. Find out which salt or ion, from a selection provided, is the best catalyst for the reaction. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride, alcl3, and. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind. Aluminum Foil And Hydrochloric Acid Equation.
From www.coursehero.com
[Solved] 3. Aluminum and hydrochloric acid react according to the Aluminum Foil And Hydrochloric Acid Equation Aluminum acts as the reducing agent, giving up electrons: When aluminum reacts with hydrochloric acid, aluminum chloride and hydrogen gas are produced. Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction of. In this problem, the salt produced will be aluminum chloride, or alcl3. Aluminium metal will react with dilute. Aluminum Foil And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED Write the equations for the following reactionsa.) Aluminium Aluminum Foil And Hydrochloric Acid Equation Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. The aluminum comes from the aluminum foil,. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the reaction.the reaction. Aluminum Foil And Hydrochloric Acid Equation.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Aluminum Foil And Hydrochloric Acid Equation In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Explore the reactions between aluminium foil and hydrochloric acid using different catalysts. 2al + 6hcl → 2alcl3 + 3h2 ↑. The reaction is irreversible as. Aluminum Foil And Hydrochloric Acid Equation.