Calorimetry Chemistry Questions . Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calculate the amount of heat needed. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the.
from www.vedantu.com
This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Calculate the amount of heat needed. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the.
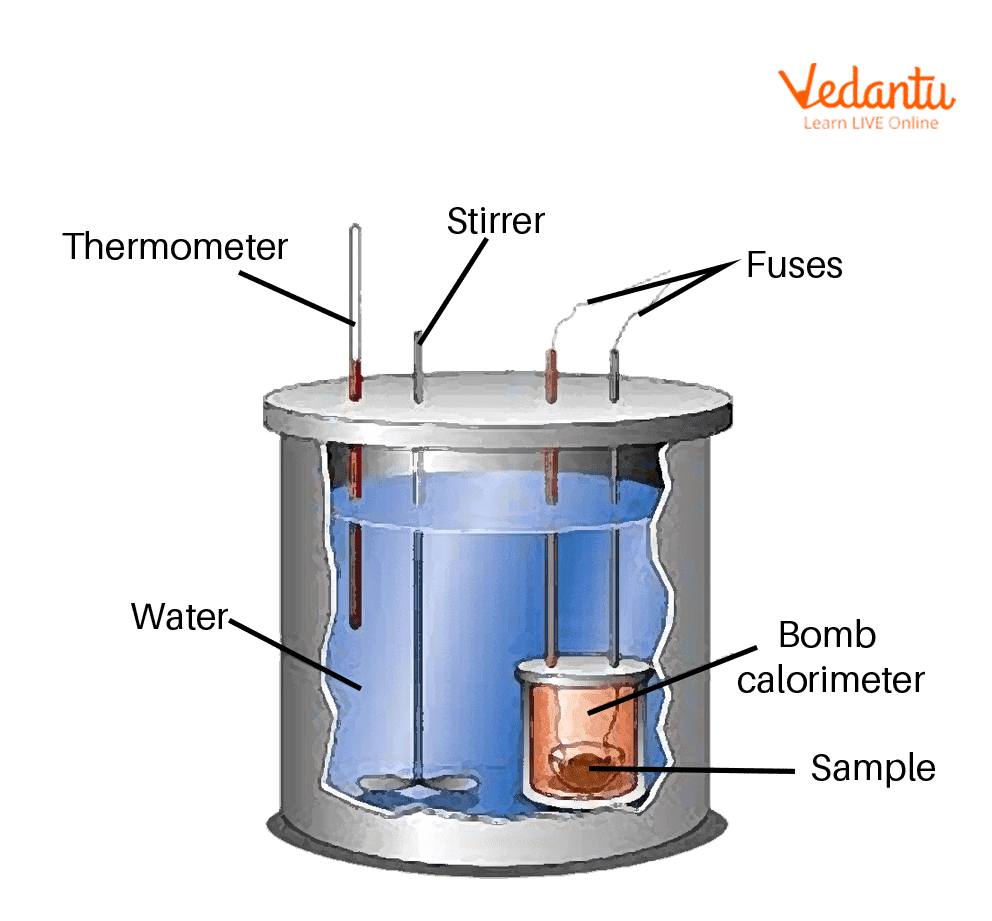
Bomb Calorimeter Learn Important Terms and Concepts
Calorimetry Chemistry Questions Calculate the amount of heat needed. Calculate the amount of heat needed. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly. Calorimetry Chemistry Questions.
From studylib.net
Calorimetry practice Calorimetry Chemistry Questions Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed. Calorimetry Chemistry Questions.
From hxeenedvw.blob.core.windows.net
Calorimeter Chemistry Ia at Beverly McPhee blog Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly. Calorimetry Chemistry Questions.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Chemistry Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on. Calorimetry Chemistry Questions.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Check your understanding of heat capacity and calorimetry. Calorimetry Chemistry Questions.
From www.revisely.co.uk
ALevel AQA Chemistry Questions Calorimetry Revisely Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. In this set of practice questions, we will go over. Calorimetry Chemistry Questions.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. When 1.0 g of fructose, c 6 h 12 o 6 (s), a. Calorimetry Chemistry Questions.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Chemistry Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Calculate the amount of heat. Calorimetry Chemistry Questions.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Chemistry Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calculate the amount of heat needed. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of. Calorimetry Chemistry Questions.
From studylib.net
Calorimetry Worksheet Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. In this set of practice. Calorimetry Chemistry Questions.
From www.youtube.com
Bomb Calorimetry Problem (Chemistry) YouTube Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. This online quiz is intended to give you extra practice. Calorimetry Chemistry Questions.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Chemistry Questions Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Check your understanding of heat capacity and calorimetry in this set of free. Calorimetry Chemistry Questions.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. This online quiz is intended to give you. Calorimetry Chemistry Questions.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Chemistry Questions Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options. Calorimetry Chemistry Questions.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. When 1.0 g of. Calorimetry Chemistry Questions.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Chemistry Questions Calculate the amount of heat needed. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Check your. Calorimetry Chemistry Questions.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Chemistry Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Calculate the amount of heat needed. When 1.0 g of. Calorimetry Chemistry Questions.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. In this set of practice questions, we will. Calorimetry Chemistry Questions.
From www.pinterest.com
Calorimetry Worksheet Answer Key Foothill High School Chemistry Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Check your understanding of heat. Calorimetry Chemistry Questions.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Calculate the amount of heat needed. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and. Calorimetry Chemistry Questions.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Use our revision notes on calorimetry for a level chemistry to understand how to measure. Calorimetry Chemistry Questions.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry. Calorimetry Chemistry Questions.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.0 g of fructose, c 6 h 12 o. Calorimetry Chemistry Questions.
From www.chegg.com
CALORIMETRY EXPERIMENT PRELAB... Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. This online quiz is intended to give you. Calorimetry Chemistry Questions.
From www.youtube.com
General Chemistry II Solving Calorimetry Problems Neutralization Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Calculate the amount of heat needed. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of. Calorimetry Chemistry Questions.
From quizzlibraryzimmer.z13.web.core.windows.net
Calorimetry Questions And Answers Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Check your understanding of heat. Calorimetry Chemistry Questions.
From studylib.net
CALORIMETRY WORKSHEET 2 Calorimetry Chemistry Questions Calculate the amount of heat needed. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. Aluminum metal can be recycled from scrap metal by. Calorimetry Chemistry Questions.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt). Calorimetry Chemistry Questions.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Chemistry Questions Calculate the amount of heat needed. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.0 g of fructose, c 6 h 12. Calorimetry Chemistry Questions.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Chemistry Questions Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calculate the amount of heat needed. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. This online. Calorimetry Chemistry Questions.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Chemistry Questions Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. Calculate the amount of heat needed. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. In this set of practice questions, we will go. Calorimetry Chemistry Questions.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Calorimetry Chemistry Questions When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. In this set of practice questions, we will. Calorimetry Chemistry Questions.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Chemistry Questions Calculate the amount of heat needed. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Check your understanding of heat capacity and calorimetry in this set of free practice questions designed for ap chemistry students. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned. Calorimetry Chemistry Questions.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Chemistry Questions This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and temperature. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Aluminum metal can be recycled from scrap metal by melting the metal to. Calorimetry Chemistry Questions.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Chemistry Questions Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units of heat and. Calorimetry Chemistry Questions.