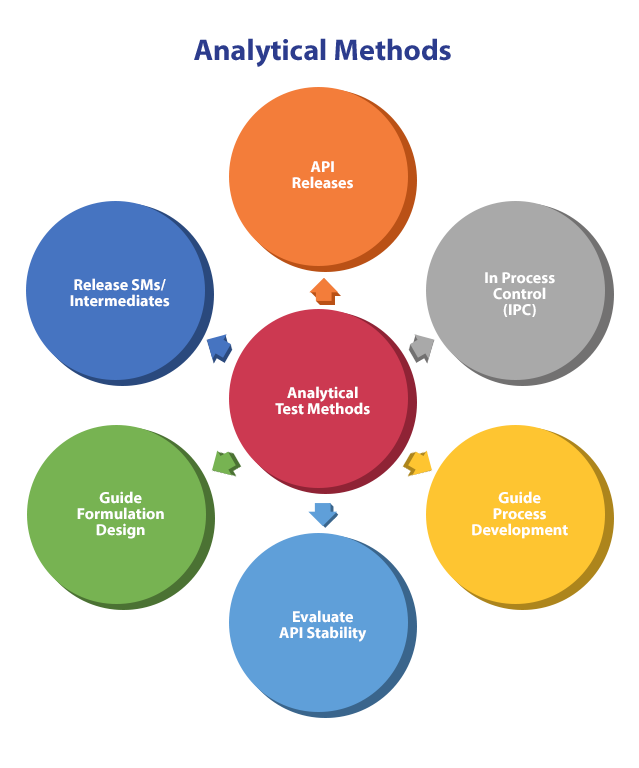
Analytical Chemistry Method Development . I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of.
from www.registech.com
Find out how to understand the sample, define. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. I) better understanding and control over critical variables;
Analytical Strategies from Early Development to Validation Part One
Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. I) better understanding and control over critical variables; Find out how to understand the sample, define. Ii) beyond traditional approach of. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit.
From www.researchgate.net
(PDF) A Comprehensive Review on HPLC Method Development, Validation Analytical Chemistry Method Development I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method. Analytical Chemistry Method Development.
From www.researchgate.net
(PDF) Key Aspects of Analytical Method Development and Validation Analytical Chemistry Method Development Ii) beyond traditional approach of. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations on how to submit analytical procedures and methods. Analytical Chemistry Method Development.
From benthambooks.com
Advanced Techniques of Analytical Chemistry Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. I) better understanding and control over critical variables; Find out how to understand the sample, define. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations on how to submit analytical. Analytical Chemistry Method Development.
From lubrizolcdmo.com
The Guide to Analytical Method Development LLS Health CDMO Analytical Chemistry Method Development I) better understanding and control over critical variables; Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method. Analytical Chemistry Method Development.
From thespectroscopy.com
Free Download General Analytical Chemistry Separation and Spectral Analytical Chemistry Method Development I) better understanding and control over critical variables; Ii) beyond traditional approach of. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography. Analytical Chemistry Method Development.
From www.researchgate.net
(PDF) A REVIEW ON BIOANALYTICAL METHOD DEVELOPMENT AND VALIDATION BY RP Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. I). Analytical Chemistry Method Development.
From lubrizolcdmo.com
The Guide to Analytical Method Development LLS Health CDMO Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Find out how to understand the sample, define. Ii) beyond traditional approach of. Learn the basic principles and steps of developing a method for liquid chromatography. Analytical Chemistry Method Development.
From studylib.net
Introduction to Analytical Chemistry Analytical Chemistry Method Development I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Ii) beyond traditional approach of. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method for liquid chromatography. Analytical Chemistry Method Development.
From veeprho.com
Analytical Method Development Forced Degradation Study Veeprho Analytical Chemistry Method Development I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development. Analytical Chemistry Method Development.
From www.researchgate.net
Principles of the green analytical chemistry for sample preparation and Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Ii) beyond traditional approach of. I). Analytical Chemistry Method Development.
From www.semanticscholar.org
Figure 2 from A Review on Novel Analytical Techniques Used in Method Analytical Chemistry Method Development Find out how to understand the sample, define. I) better understanding and control over critical variables; Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which. Analytical Chemistry Method Development.
From kingkagsblog.com
Why Is Analytical Method Validation Important? Analytical Chemistry Method Development Ii) beyond traditional approach of. I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and. Analytical Chemistry Method Development.
From hoahoc.org
What is Analytical Chemistry? Analytical Chemistry Method Development Find out how to understand the sample, define. Ii) beyond traditional approach of. I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and. Analytical Chemistry Method Development.
From pubs.acs.org
Selection of Analytical Technology and Development of Analytical Analytical Chemistry Method Development I) better understanding and control over critical variables; Find out how to understand the sample, define. Ii) beyond traditional approach of. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations. Analytical Chemistry Method Development.
From www.registech.com
Exploring Analytical Method Development for Drug Substances Regis Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. Find out how to understand the sample, define. This guidance provides recommendations on how to submit analytical procedures and methods. Analytical Chemistry Method Development.
From www.qlaboratories.com
Analytical Chemistry Laboratory Services Q Laboratories Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Find out how to understand the sample, define. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Ii) beyond traditional approach of. I) better understanding and control over critical variables;. Analytical Chemistry Method Development.
From dheerajpratapsingh0.blogspot.com
Steps for HPLC Method Development Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development. Analytical Chemistry Method Development.
From www.registech.com
Analytical Strategies from Early Development to Validation Part One Analytical Chemistry Method Development I) better understanding and control over critical variables; Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations. Analytical Chemistry Method Development.
From www.slideshare.net
Analytical method development Analytical Chemistry Method Development Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the. Analytical Chemistry Method Development.
From www.linkedin.com
Analytical Method Development Analytical Chemistry Method Development Ii) beyond traditional approach of. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. I) better understanding and control over critical variables; Find out how to understand the sample, define. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations. Analytical Chemistry Method Development.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Analytical Chemistry Method Development I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method. Analytical Chemistry Method Development.
From www.morebooks.de
Analytical method development and validation by uv and hplc techniques Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Ii) beyond traditional approach of. I) better understanding and control over critical variables;. Analytical Chemistry Method Development.
From studylib.net
Analytical Method Development Analytical Chemistry Method Development Find out how to understand the sample, define. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. I) better understanding and control over critical variables; Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which. Analytical Chemistry Method Development.
From www.linkedin.com
ANALYTICAL METHODS DEVELOPMENT Analytical Chemistry Method Development Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. I). Analytical Chemistry Method Development.
From www.sofpromed.com
An Introduction to Analytical Method Development and Validation in Analytical Chemistry Method Development Ii) beyond traditional approach of. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Find out how to understand the sample, define. I) better understanding and control over critical variables; Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations. Analytical Chemistry Method Development.
From www.studocu.com
Introduction To Analytical Chemistry INTRODUCTION TO ANALYTICAL Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. I) better understanding and control over. Analytical Chemistry Method Development.
From www.youtube.com
Column Chemistry Column Selection HPLC Analytical Method Analytical Chemistry Method Development Find out how to understand the sample, define. Ii) beyond traditional approach of. I) better understanding and control over critical variables; This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis.. Analytical Chemistry Method Development.
From altusformulation.com
What are analytical method development services? Analytical Chemistry Method Development This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development. Analytical Chemistry Method Development.
From www.researchgate.net
(PDF) Method Development and Validation of Residual Solvents in Analytical Chemistry Method Development I) better understanding and control over critical variables; Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and. Analytical Chemistry Method Development.
From www.slideserve.com
PPT ANALYTICAL CHEMISTRY PowerPoint Presentation, free download ID Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. I) better understanding and control over critical variables; Find out how to understand the sample, define. This guidance provides recommendations. Analytical Chemistry Method Development.
From abzena.com
Analytical Chemistry Method Development Drug Product Characterization Analytical Chemistry Method Development Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. Ii) beyond traditional approach of. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. I) better understanding and control over critical variables;. Analytical Chemistry Method Development.
From www.researchgate.net
(PDF) Chemometric Strategies for Fully Automated Interpretive Method Analytical Chemistry Method Development Find out how to understand the sample, define. Ii) beyond traditional approach of. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. I) better understanding and control over critical variables; Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis.. Analytical Chemistry Method Development.
From abzena.com
Chemistry Bioconjugates & Chemistry Chemistry Capabilities Analytical Chemistry Method Development Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. Ii) beyond traditional approach of. Find out how to understand the sample, define. Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis. This guidance provides recommendations on how to submit analytical procedures and methods. Analytical Chemistry Method Development.
From pubs.rsc.org
Quality by design approach for green HPLC method development for Analytical Chemistry Method Development Find out how to understand the sample, define. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Ii) beyond traditional approach of. I) better understanding and control over critical variables; Learn the basic principles and steps of developing a method for liquid chromatography (lc), mass spectrometry (ms), or thermal analysis.. Analytical Chemistry Method Development.
From www.researchgate.net
(PDF) A Stability Demonstrating HPLC Method Development and Validation Analytical Chemistry Method Development Enhanced pharmaceutical development approaches, which apply qbd concepts, rely heavily on the development of fit. This guidance provides recommendations on how to submit analytical procedures and methods validation data for drugs and biologics applications. Find out how to understand the sample, define. Ii) beyond traditional approach of. Learn the basic principles and steps of developing a method for liquid chromatography. Analytical Chemistry Method Development.