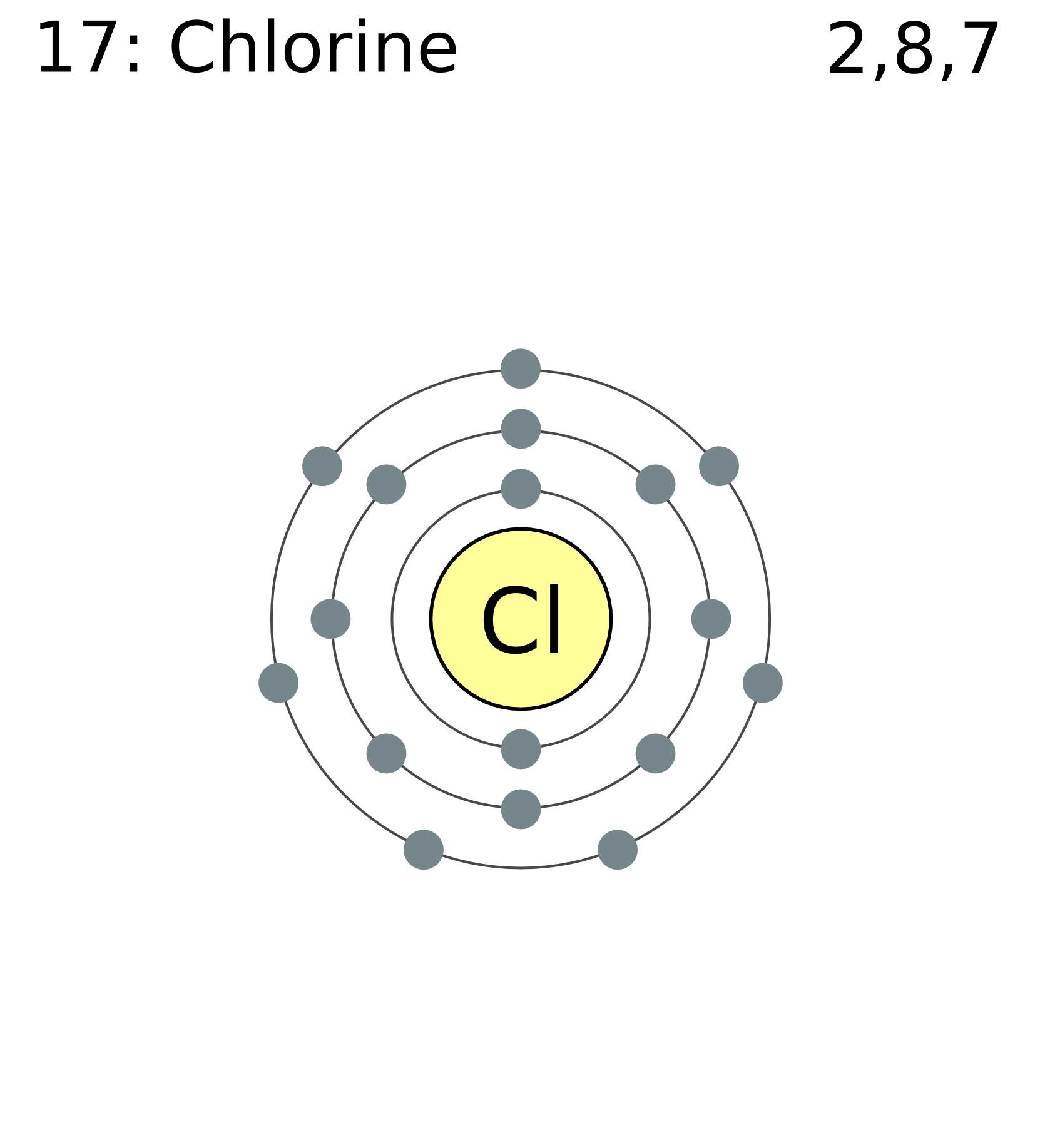
Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond . Determine the total number of valence electrons in chlorine molecule. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. One short of 8, which. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The electron configuration shows that the last shell. the total number of electrons in a valence shell is called valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is.
from commons.wikimedia.org
the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. The electron configuration shows that the last shell. The chlorine molecule contains two chlorine atoms. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. the total number of electrons in a valence shell is called valence electrons. In the periodic table, chlorine is. One short of 8, which. Determine the total number of valence electrons in chlorine molecule.
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond One short of 8, which. In the periodic table, chlorine is. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Determine the total number of valence electrons in chlorine molecule. the total number of electrons in a valence shell is called valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. One short of 8, which. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. The chlorine molecule contains two chlorine atoms. The electron configuration shows that the last shell.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. Determine the total number of valence electrons in chlorine molecule. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. One short of 8, which. the total number of electrons. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the total number of electrons in a valence shell is called. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the total number of electrons in a valence shell is called valence electrons. The electron configuration shows that the last shell. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. One. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. The electron configuration shows that the last shell. The chlorine molecule contains two chlorine atoms. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. The electron configuration shows that the last shell. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the total number of electrons in a valence shell is called valence electrons. The chlorine molecule. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From slideplayer.com
Science 20 Unit A CHEMISTRY ppt download Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the total number of electrons in a valence shell is called valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. the 3s^2 3p^5 electrons are the outermost electrons, so. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of electrons in a valence shell is called valence electrons. 93 rows you may assume the valences of the chemical elements—the number of. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The electron configuration shows that the last shell. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is. One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. . Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. One short of 8, which. The chlorine molecule contains two chlorine atoms. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Determine the total number of valence electrons in chlorine molecule. the. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From quizdislodging.z4.web.core.windows.net
What Element Has 7 Electrons Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. The chlorine molecule contains two chlorine atoms. The electron configuration shows that the last shell. Determine the total number of valence electrons in chlorine molecule. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. the 3s^2 3p^5 electrons are the outermost electrons, so. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From guidefixbukukurj.z21.web.core.windows.net
Bohr Classification Of Elements Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond One short of 8, which. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. In the periodic table, chlorine is. The chlorine molecule contains two chlorine atoms. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the total number of electrons in a valence. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. The electron configuration shows that the last shell. One short of 8, which. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of electrons in a valence shell is called valence electrons. Determine the total number of valence electrons in chlorine molecule. Chlorine, with. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From slideplayer.com
Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and ppt Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond The electron configuration shows that the last shell. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. In the periodic table, chlorine is. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond Determine the total number of valence electrons in chlorine molecule. One short of 8, which. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. the total number of electrons in a valence shell is called valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the total number of electrons in a valence shell is called valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. The chlorine molecule contains two chlorine atoms. Chlorine, with 7. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the total number of electrons in a valence shell is called valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. One short of 8, which. The electron configuration shows that the last shell.. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond Determine the total number of valence electrons in chlorine molecule. The chlorine molecule contains two chlorine atoms. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. In the periodic table, chlorine is. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. One short of 8, which. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of electrons in a valence shell is called valence electrons. The electron configuration shows that the last shell. 93 rows you may assume the valences of the chemical elements—the. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond Determine the total number of valence electrons in chlorine molecule. the total number of electrons in a valence shell is called valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. One short of 8, which. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the total number of electrons in a valence shell is called valence electrons. One short of 8, which. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. The chlorine molecule contains two chlorine atoms. In the periodic. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.teachoo.com
Uses of Metals and Non Metals [in Daily life] Teachoo Concepts Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond The electron configuration shows that the last shell. One short of 8, which. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From slideplayer.com
Periodic Table, Part 2 (Chapter 5) ppt download Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond One short of 8, which. The electron configuration shows that the last shell. Determine the total number of valence electrons in chlorine molecule. In the periodic table, chlorine is. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. The chlorine molecule contains two chlorine atoms. The electron configuration shows that the last shell. One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond In the periodic table, chlorine is. the total number of electrons in a valence shell is called valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. One short of 8, which.. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond The electron configuration shows that the last shell. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the total number of electrons in a valence shell is called valence electrons. In the periodic table, chlorine is. One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.slideshare.net
Elements and Chemical Bonds Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Determine the total number of valence electrons in chlorine molecule. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. The electron configuration shows that the last shell. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. One short. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. In the periodic table, chlorine is. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The chlorine molecule. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. Determine the total number of valence electrons in chlorine molecule. the total number of electrons in a valence shell is called valence electrons. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. One short of 8, which.. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From philschatz.com
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond The chlorine molecule contains two chlorine atoms. The electron configuration shows that the last shell. One short of 8, which. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. In the periodic table, chlorine is. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. . Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond The chlorine molecule contains two chlorine atoms. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. In the periodic table, chlorine is. the central atom, boron, contributes three. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond One short of 8, which. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. The electron configuration shows that the last shell. the total number of electrons in a valence shell is called valence electrons. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From littleeagles.edu.vn
How To Find The Protons, Neutrons And Electrons For Chlorine? Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond One short of 8, which. Chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Determine the total number of valence electrons in chlorine molecule. the total number of electrons in a valence shell is called valence electrons.. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.
From www.researchgate.net
hypochlorous acid Chlorine has 7 valence electrons, oxygen has 6 Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond Determine the total number of valence electrons in chlorine molecule. In the periodic table, chlorine is. The chlorine molecule contains two chlorine atoms. One short of 8, which. the total number of electrons in a valence shell is called valence electrons. the central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The. Chlorine Has 7 Valence Electrons With What Group Of Elements Will Chlorine Most Readily Bond.