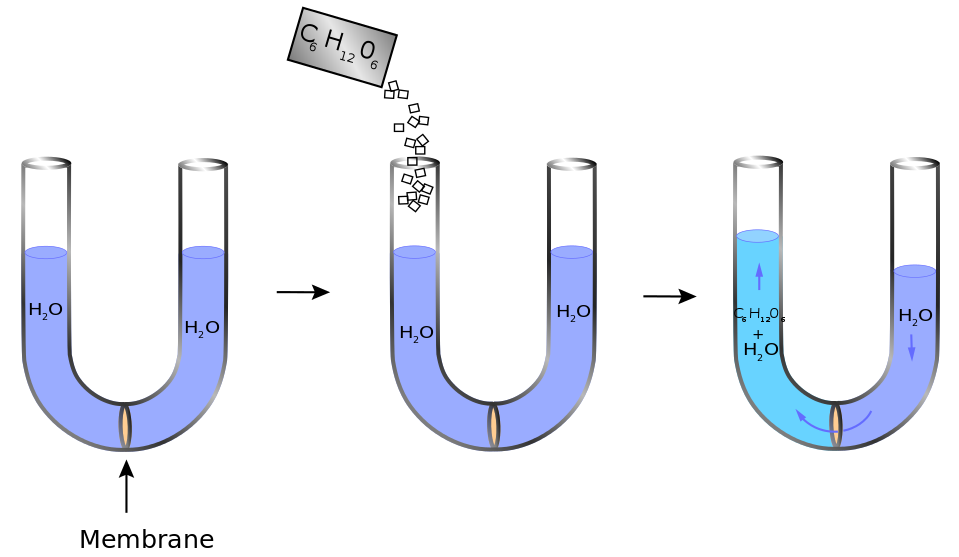
Osmometer Vs Osmolality . osmolality and tonicity: the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. Will i need to do anything. the mean difference in osmolarity was 0 ± 2 mmol/l. Osmolality is a property of a particular solution and is. Why do i need an osmolality test? What happens during an osmolality test? Whole blood was greater than plasma in 90 of 168. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. osmolality is the concentration of osmoles in a mass of solvent. what are they used for? They measure the osmolality of a liquid sample by comparing a particular. In biologic systems, osmolality is expressed as. most modern osmometers share a common principle:
from pediaa.com
Whole blood was greater than plasma in 90 of 168. Why do i need an osmolality test? They measure the osmolality of a liquid sample by comparing a particular. what are they used for? osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. osmolality and tonicity: osmolality is the concentration of osmoles in a mass of solvent. the mean difference in osmolarity was 0 ± 2 mmol/l. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. Will i need to do anything.
Difference Between Osmolarity and Osmolality Definition, Explanation
Osmometer Vs Osmolality Will i need to do anything. They measure the osmolality of a liquid sample by comparing a particular. Whole blood was greater than plasma in 90 of 168. In biologic systems, osmolality is expressed as. osmolality is the concentration of osmoles in a mass of solvent. the mean difference in osmolarity was 0 ± 2 mmol/l. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. most modern osmometers share a common principle: Osmolality is a property of a particular solution and is. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Will i need to do anything. what are they used for? What happens during an osmolality test? Why do i need an osmolality test? osmolality and tonicity:
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. In biologic systems, osmolality is expressed as. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Whole blood was greater than plasma in 90 of 168. what are they used for? the osmolality of a real solution. Osmometer Vs Osmolality.
From www.slideserve.com
PPT Renal Review PowerPoint Presentation ID1400305 Osmometer Vs Osmolality In biologic systems, osmolality is expressed as. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. most modern osmometers share a common principle: the mean difference in osmolarity was 0 ± 2 mmol/l. Whole blood was greater than plasma in 90 of 168. Osmolality is a property of. Osmometer Vs Osmolality.
From healthjade.net
Osmolar gap, osmolar gap equation, normal osmolar gap Osmometer Vs Osmolality osmolality is the concentration of osmoles in a mass of solvent. Whole blood was greater than plasma in 90 of 168. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. Will i need to do anything. In biologic systems, osmolality is expressed as. They measure the osmolality. Osmometer Vs Osmolality.
From www.slideserve.com
PPT Tonicity, Osmoticity , Osmolarity , & Osmolality PowerPoint Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. most modern osmometers share a common principle: They measure the osmolality of a liquid sample by comparing a particular. osmolality and tonicity: Whole blood was greater than plasma in 90 of 168. osmolality is a colligative property of solutions that depends on the number of dissolved. Osmometer Vs Osmolality.
From www.il-biosystems.com
OsmoPRO MAX Osmometer Efficient Osmolality Determination Osmometer Vs Osmolality osmolality is the concentration of osmoles in a mass of solvent. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Whole blood was greater than plasma in 90 of 168. Osmolality is a property of a particular solution and is. the mean difference in osmolarity was 0 ±. Osmometer Vs Osmolality.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmometer Vs Osmolality They measure the osmolality of a liquid sample by comparing a particular. Why do i need an osmolality test? osmolality is the concentration of osmoles in a mass of solvent. osmolality and tonicity: What happens during an osmolality test? In biologic systems, osmolality is expressed as. osmolality is a colligative property of solutions that depends on the. Osmometer Vs Osmolality.
From www.concordscientificdevices.com
vaporpressureosmometer Osmometer Vs Osmolality osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. osmolality and tonicity: Osmolality is a property of a particular solution and is. They measure the osmolality of a liquid sample by comparing a particular. what are they used for? osmolality is the concentration of osmoles in a. Osmometer Vs Osmolality.
From www.iconsci.com
What Are Osmometers? Osmometer Vs Osmolality They measure the osmolality of a liquid sample by comparing a particular. Whole blood was greater than plasma in 90 of 168. most modern osmometers share a common principle: osmolality is the concentration of osmoles in a mass of solvent. the mean difference in osmolarity was 0 ± 2 mmol/l. Will i need to do anything. What. Osmometer Vs Osmolality.
From askanydifference.com
Osmolality vs Osmolarity Difference and Comparison Osmometer Vs Osmolality Why do i need an osmolality test? Will i need to do anything. the mean difference in osmolarity was 0 ± 2 mmol/l. most modern osmometers share a common principle: the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. What happens during an osmolality test? In. Osmometer Vs Osmolality.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 Osmometer Vs Osmolality the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. osmolality and tonicity: Why do i need an osmolality test? In biologic systems, osmolality is expressed as. what are they used for? Whole blood was greater than plasma in 90 of 168. osmolality is the concentration. Osmometer Vs Osmolality.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality Osmometer Vs Osmolality osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. osmolality and tonicity: the mean difference in osmolarity was 0 ± 2 mmol/l. In biologic systems, osmolality is expressed as. what are they used for? They measure the osmolality of a liquid sample by comparing a particular. . Osmometer Vs Osmolality.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. most modern osmometers share a common principle: Whole blood was greater than plasma in 90 of 168. osmolality and tonicity: osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. In biologic systems, osmolality is expressed as. Why. Osmometer Vs Osmolality.
From www.researchgate.net
Plots of osmolality by vapor pressure (left) and freezing point Osmometer Vs Osmolality osmolality is the concentration of osmoles in a mass of solvent. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is a property of a particular solution and is. Why do i need an osmolality test? most modern osmometers share a common principle: They measure the osmolality. Osmometer Vs Osmolality.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical Osmometer Vs Osmolality What happens during an osmolality test? most modern osmometers share a common principle: osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Why do i need an osmolality test? Osmolality is a property of a particular solution and is. Whole blood was greater than plasma in 90 of 168.. Osmometer Vs Osmolality.
From dxokrvgoz.blob.core.windows.net
Osmolality Stool Test at Samuel Mundy blog Osmometer Vs Osmolality In biologic systems, osmolality is expressed as. the mean difference in osmolarity was 0 ± 2 mmol/l. Why do i need an osmolality test? osmolality is the concentration of osmoles in a mass of solvent. What happens during an osmolality test? Whole blood was greater than plasma in 90 of 168. They measure the osmolality of a liquid. Osmometer Vs Osmolality.
From www.il-biosystems.com
OsmoPRO MAX Osmometer Efficient Osmolality Determination Osmometer Vs Osmolality most modern osmometers share a common principle: Why do i need an osmolality test? osmolality and tonicity: What happens during an osmolality test? They measure the osmolality of a liquid sample by comparing a particular. Osmolality is a property of a particular solution and is. what are they used for? the mean difference in osmolarity was. Osmometer Vs Osmolality.
From www.pinterest.ca
Osmolality Nursing school tips, Nursing mnemonics, Nursing information Osmometer Vs Osmolality Osmolality is a property of a particular solution and is. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Whole blood was greater than plasma in 90 of 168. What happens during an osmolality test? Why do i need an osmolality test? what are they used for? They measure. Osmometer Vs Osmolality.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmometer Vs Osmolality What happens during an osmolality test? Whole blood was greater than plasma in 90 of 168. Will i need to do anything. Why do i need an osmolality test? In biologic systems, osmolality is expressed as. osmolality is the concentration of osmoles in a mass of solvent. most modern osmometers share a common principle: the mean difference. Osmometer Vs Osmolality.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki Osmometer Vs Osmolality What happens during an osmolality test? osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. most modern osmometers share a common principle: In biologic systems, osmolality is expressed as. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is. Osmometer Vs Osmolality.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. most modern osmometers share a common principle: In biologic systems, osmolality is expressed as. Whole blood was greater than plasma in 90 of 168. osmolality and tonicity: They. Osmometer Vs Osmolality.
From www.physiologyweb.com
Physiology Graph Increases in plasma osmolality increase antidiuretic Osmometer Vs Osmolality osmolality is the concentration of osmoles in a mass of solvent. what are they used for? most modern osmometers share a common principle: In biologic systems, osmolality is expressed as. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. the mean difference in osmolarity. Osmometer Vs Osmolality.
From www.biophlox.com
A Complete Lab Guide to Osmometer Osmometer Vs Osmolality Why do i need an osmolality test? osmolality is the concentration of osmoles in a mass of solvent. Will i need to do anything. most modern osmometers share a common principle: What happens during an osmolality test? In biologic systems, osmolality is expressed as. osmolality and tonicity: what are they used for? the mean difference. Osmometer Vs Osmolality.
From drawittoknowit.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is a property of a particular solution and is. Whole blood was greater than plasma in 90 of 168. most modern osmometers share a common principle: Will i. Osmometer Vs Osmolality.
From dxotfwlri.blob.core.windows.net
Lab Test Osmolality Low at Elizabeth Sauer blog Osmometer Vs Osmolality Will i need to do anything. Osmolality is a property of a particular solution and is. They measure the osmolality of a liquid sample by comparing a particular. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. osmolality and tonicity: osmolality is a colligative property of. Osmometer Vs Osmolality.
From droualb.faculty.mjc.edu
Chapter 4 Osmometer Vs Osmolality the mean difference in osmolarity was 0 ± 2 mmol/l. osmolality and tonicity: most modern osmometers share a common principle: the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. what are they used for? osmolality is the concentration of osmoles in a mass. Osmometer Vs Osmolality.
From slideplayer.com
Fluid and electrolyte balance ppt download Osmometer Vs Osmolality They measure the osmolality of a liquid sample by comparing a particular. most modern osmometers share a common principle: the mean difference in osmolarity was 0 ± 2 mmol/l. What happens during an osmolality test? Will i need to do anything. osmolality is a colligative property of solutions that depends on the number of dissolved particles in. Osmometer Vs Osmolality.
From studylib.net
Tonicity, Osmoticity, Osmolarity, & Osmolality Osmometer Vs Osmolality Osmolality is a property of a particular solution and is. Whole blood was greater than plasma in 90 of 168. osmolality and tonicity: Why do i need an osmolality test? Will i need to do anything. what are they used for? the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating. Osmometer Vs Osmolality.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the Osmometer Vs Osmolality most modern osmometers share a common principle: Whole blood was greater than plasma in 90 of 168. the mean difference in osmolarity was 0 ± 2 mmol/l. Osmolality is a property of a particular solution and is. osmolality and tonicity: They measure the osmolality of a liquid sample by comparing a particular. what are they used. Osmometer Vs Osmolality.
From www.slideserve.com
PPT Physiology of vasopressin secretion THE antidiuretic hormone Osmometer Vs Osmolality In biologic systems, osmolality is expressed as. Osmolality is a property of a particular solution and is. osmolality and tonicity: Why do i need an osmolality test? What happens during an osmolality test? most modern osmometers share a common principle: what are they used for? osmolality is the concentration of osmoles in a mass of solvent.. Osmometer Vs Osmolality.
From www.difference.wiki
Osmolarity vs. Osmolality What’s the Difference? Osmometer Vs Osmolality the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. In biologic systems, osmolality is expressed as. They measure the osmolality of a liquid sample by comparing a particular. Whole blood was greater than plasma in 90 of 168. osmolality is a colligative property of solutions that depends. Osmometer Vs Osmolality.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmometer Vs Osmolality osmolality is the concentration of osmoles in a mass of solvent. Whole blood was greater than plasma in 90 of 168. What happens during an osmolality test? the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. osmolality is a colligative property of solutions that depends on. Osmometer Vs Osmolality.
From www.il-biosystems.com
Osmo1 Osmometer Measuring osmolality quickly and easily Osmometer Vs Osmolality what are they used for? osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is a property of a particular solution and is. most modern osmometers share a common principle: They measure the osmolality of a liquid sample by comparing a particular. Why do i need an. Osmometer Vs Osmolality.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality Osmometer Vs Osmolality Whole blood was greater than plasma in 90 of 168. osmolality is the concentration of osmoles in a mass of solvent. osmolality and tonicity: Will i need to do anything. Osmolality is a property of a particular solution and is. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes. Osmometer Vs Osmolality.
From www.thoughtco.com
Osmotic Pressure and Tonicity Osmometer Vs Osmolality They measure the osmolality of a liquid sample by comparing a particular. osmolality is the concentration of osmoles in a mass of solvent. osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Will i need to do anything. what are they used for? Whole blood was greater than. Osmometer Vs Osmolality.
From www.slideserve.com
PPT IV Therapy PowerPoint Presentation, free download ID9628818 Osmometer Vs Osmolality osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Will i need to do anything. In biologic systems, osmolality is expressed as. Why do i need an osmolality test? Whole blood was greater than plasma in 90 of 168. the mean difference in osmolarity was 0 ± 2 mmol/l.. Osmometer Vs Osmolality.