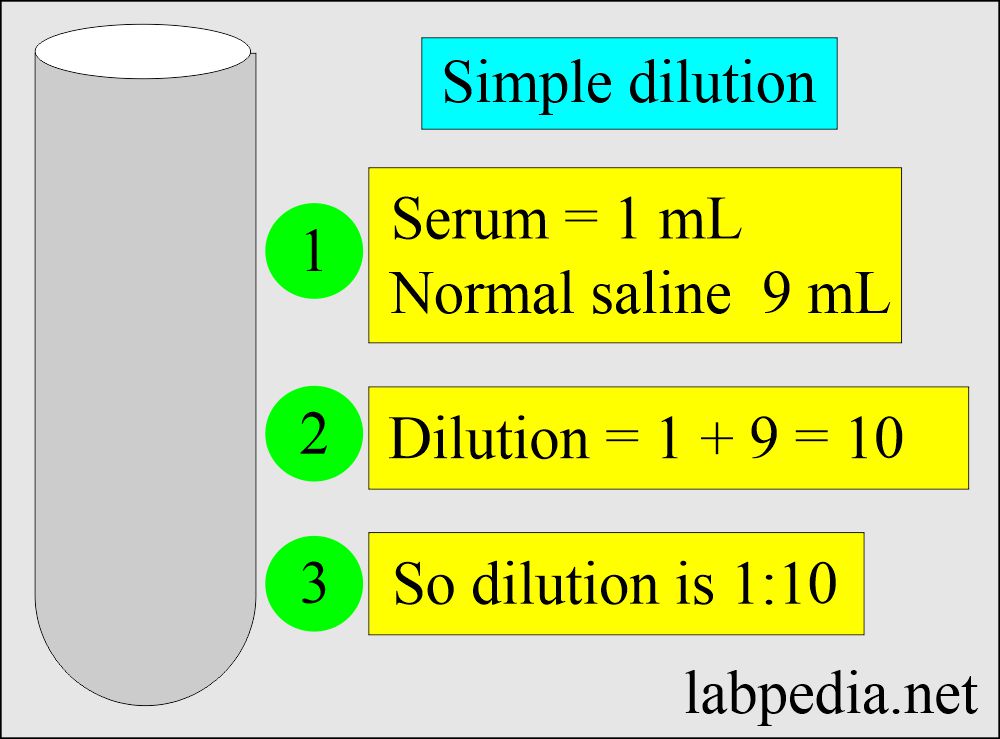
Solutions And Dilutions Lab . explain how concentrations can be changed in the lab. understand how stock solutions are used in the laboratory. Concentration in moles per litre, molar concentration or molarity. preparing solutions by dilution. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. • use volumetric and mohr pipets and a volumetric flask. • prepare a dilute solution from a more concentrated one. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution of solutions. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Understand how stock solutions are used in the laboratory.
from labpedia.net
this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Understand how stock solutions are used in the laboratory. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. • prepare a dilute solution from a more concentrated one. Concentration in moles per litre, molar concentration or molarity. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. • use volumetric and mohr pipets and a volumetric flask.
Solutions Part 1 Solutions Preparation used in Clinical Laboratory
Solutions And Dilutions Lab Concentration in moles per litre, molar concentration or molarity. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. explain how concentrations can be changed in the lab. understand how stock solutions are used in the laboratory. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. • use volumetric and mohr pipets and a volumetric flask. Understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. dilution of solutions. • prepare a dilute solution from a more concentrated one. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Concentration in moles per litre, molar concentration or molarity. preparing solutions by dilution.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Solutions And Dilutions Lab explain how concentrations can be changed in the lab. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. preparing solutions by dilution. Understand how stock solutions are. Solutions And Dilutions Lab.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solutions And Dilutions Lab preparing solutions by dilution. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the process whereby the concentration of a solution is lessened by the addition. Solutions And Dilutions Lab.
From dxoxgrbcm.blob.core.windows.net
Dilution Testing For Drugs at Ernesto Anderson blog Solutions And Dilutions Lab Concentration in moles per litre, molar concentration or molarity. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. • prepare a dilute solution from a more concentrated one. For. Solutions And Dilutions Lab.
From studylib.net
Solution Dilution Lab Solutions And Dilutions Lab • prepare a dilute solution from a more concentrated one. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Understand how stock solutions are used in the laboratory. • use volumetric and mohr pipets and a volumetric flask. Concentration in moles per litre, molar concentration or molarity. Dilution is the. Solutions And Dilutions Lab.
From www.chegg.com
Solved LAB 2 Solutions & Dilutions Prelaboratory Solutions And Dilutions Lab • use volumetric and mohr pipets and a volumetric flask. dilution of solutions. • prepare a dilute solution from a more concentrated one. understand how stock solutions are used in the laboratory. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Understand how stock. Solutions And Dilutions Lab.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Solutions And Dilutions Lab Understand how stock solutions are used in the laboratory. preparing solutions by dilution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. • prepare a dilute solution from a more concentrated one. dilution of solutions. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and. Solutions And Dilutions Lab.
From www.chegg.com
Solved LAB 2 Solutions & Dilutions Prelaboratory Solutions And Dilutions Lab this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. • use volumetric and mohr pipets and a volumetric flask. understand how stock solutions are used in the laboratory. dilution of solutions. • prepare a dilute solution from a more concentrated one. two skills. Solutions And Dilutions Lab.
From www.studocu.com
BMS231 Solutions, Dilutions, and Concentrations Part 2 Lab Notes 1 Solutions And Dilutions Lab preparing solutions by dilution. Concentration in moles per litre, molar concentration or molarity. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. dilution. Solutions And Dilutions Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Solutions And Dilutions Lab Concentration in moles per litre, molar concentration or molarity. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. • prepare a dilute solution from a more concentrated one. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. dilution of solutions. preparing solutions by. Solutions And Dilutions Lab.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Solutions And Dilutions Lab For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Concentration in moles per litre, molar concentration or molarity. . Solutions And Dilutions Lab.
From www.youtube.com
Solutions and Dilutions pre lab video final YouTube Solutions And Dilutions Lab dilution of solutions. • use volumetric and mohr pipets and a volumetric flask. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. Dilution is the. Solutions And Dilutions Lab.
From www.slideserve.com
PPT Solutions & Dilutions PowerPoint Presentation, free download ID Solutions And Dilutions Lab two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. explain how concentrations can be. Solutions And Dilutions Lab.
From www.studocu.com
Pipetting Solutions and Dilutions Laboratory 22 Pipetting, Solutions Solutions And Dilutions Lab Understand how stock solutions are used in the laboratory. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. dilution of solutions. understand how stock solutions are used in the laboratory. • use volumetric and mohr pipets and a volumetric flask. Dilution is the process whereby. Solutions And Dilutions Lab.
From www.chegg.com
Lab 3 Solutions, Dilutions, Spectrometry General Solutions And Dilutions Lab Concentration in moles per litre, molar concentration or molarity. dilution of solutions. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. understand how stock solutions are used. Solutions And Dilutions Lab.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Solutions And Dilutions Lab understand how stock solutions are used in the laboratory. • prepare a dilute solution from a more concentrated one. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor.. Solutions And Dilutions Lab.
From www.studypool.com
SOLUTION Dilution laboratory Studypool Solutions And Dilutions Lab Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution of solutions. preparing solutions by dilution. • use volumetric and mohr pipets and a volumetric flask. • prepare a dilute solution from a more concentrated one. Understand how stock solutions are used in the laboratory. explain how concentrations. Solutions And Dilutions Lab.
From www.chegg.com
Solved Lab 11 Solutions and Dilutions 1. Four solutions Solutions And Dilutions Lab preparing solutions by dilution. Concentration in moles per litre, molar concentration or molarity. Understand how stock solutions are used in the laboratory. dilution of solutions. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. this video and written tutorial will guide you through how to make a dilution and how to. Solutions And Dilutions Lab.
From www.studocu.com
Solutions and Dilutions PreLab CHEM 40 Studocu Solutions And Dilutions Lab two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Understand how stock solutions are used in the laboratory. • prepare a dilute solution from a more concentrated one. this video and written tutorial will guide you through how to make a dilution and how to. Solutions And Dilutions Lab.
From www.chegg.com
Solved Solutions and Dilutions Lab 1. What part of a Solutions And Dilutions Lab Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. explain how concentrations can be changed in the lab. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Understand how stock solutions are used in the laboratory. • use volumetric and. Solutions And Dilutions Lab.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Solutions And Dilutions Lab preparing solutions by dilution. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. Understand how stock solutions are used in the laboratory. • use volumetric and mohr pipets and a volumetric flask. understand how stock solutions are used in the laboratory. • prepare a dilute solution from a more concentrated one.. Solutions And Dilutions Lab.
From chemistryknights.blogspot.com
Chemistry Knights VIDEO DILUTION LAB CALCULATIONS Solutions And Dilutions Lab preparing solutions by dilution. Concentration in moles per litre, molar concentration or molarity. understand how stock solutions are used in the laboratory. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. dilution of solutions. • prepare a dilute solution from a more concentrated one. Understand how stock. Solutions And Dilutions Lab.
From www.youtube.com
Lab Demonstration Solution Preparation & Dilution. YouTube Solutions And Dilutions Lab dilution of solutions. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. • use volumetric and mohr pipets and a volumetric flask. • prepare a dilute solution from a more concentrated one. preparing solutions by dilution. understand how stock solutions are used in the laboratory. explain. Solutions And Dilutions Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Solutions And Dilutions Lab For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. • prepare a dilute solution from a more concentrated one. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. explain how concentrations can be changed in the lab. Concentration in moles per. Solutions And Dilutions Lab.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Solutions And Dilutions Lab preparing solutions by dilution. Concentration in moles per litre, molar concentration or molarity. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. understand how stock solutions are used in the. Solutions And Dilutions Lab.
From dxobyjbye.blob.core.windows.net
How To Make Dilutions In Lab at Drew Ballard blog Solutions And Dilutions Lab Concentration in moles per litre, molar concentration or molarity. explain how concentrations can be changed in the lab. • prepare a dilute solution from a more concentrated one. Understand how stock solutions are used in the laboratory. dilution of solutions. • use volumetric and mohr pipets and a volumetric flask. this video and written tutorial will. Solutions And Dilutions Lab.
From www.studocu.com
Lab Report Solutions, Dilutions and Titrations How can solutions Solutions And Dilutions Lab two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. preparing solutions by dilution. For example, we might say that a glass of iced tea becomes increasingly diluted as the. Solutions And Dilutions Lab.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Solutions And Dilutions Lab Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. two skills that are essential to a chemist are preparing solutions of known molarity (m). Solutions And Dilutions Lab.
From dxobyjbye.blob.core.windows.net
How To Make Dilutions In Lab at Drew Ballard blog Solutions And Dilutions Lab For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. this video and written tutorial will guide you through how to make a dilution and how to calculate the. Solutions And Dilutions Lab.
From medlabstudyhall.com
Performing Dilutions for Laboratory Analysis Med Lab Study Hall Solutions And Dilutions Lab For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. two skills. Solutions And Dilutions Lab.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Solutions And Dilutions Lab two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. explain how concentrations can be changed in the lab. preparing solutions by dilution. this video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Concentration. Solutions And Dilutions Lab.
From www.chegg.com
Solved molarity, dilutions, and preparing solutions lab Solutions And Dilutions Lab explain how concentrations can be changed in the lab. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. preparing solutions by dilution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. • use volumetric and mohr pipets and a volumetric flask.. Solutions And Dilutions Lab.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Solutions And Dilutions Lab • use volumetric and mohr pipets and a volumetric flask. preparing solutions by dilution. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. this video and written tutorial will guide you through how to make a dilution and how to calculate the. Solutions And Dilutions Lab.
From studylib.net
Lab The Dilution Solution Solutions And Dilutions Lab preparing solutions by dilution. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. dilution of solutions. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and. Solutions And Dilutions Lab.
From www.youtube.com
How to Make a Dilution of a Stock Solution YouTube Solutions And Dilutions Lab two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry. • prepare a dilute solution from a more concentrated one. Concentration in. Solutions And Dilutions Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Solutions And Dilutions Lab understand how stock solutions are used in the laboratory. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting solutions of known molarity. Muriatic acid, another name for hcl (aq), is widely used. Solutions And Dilutions Lab.