How Is Atomic Size Measured . The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is difficult to. Have you ever wondered just how tiny atoms can be, or what determines their size? In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located.
from periodictableguide.com
A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is difficult to. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Have you ever wondered just how tiny atoms can be, or what determines their size? In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures.
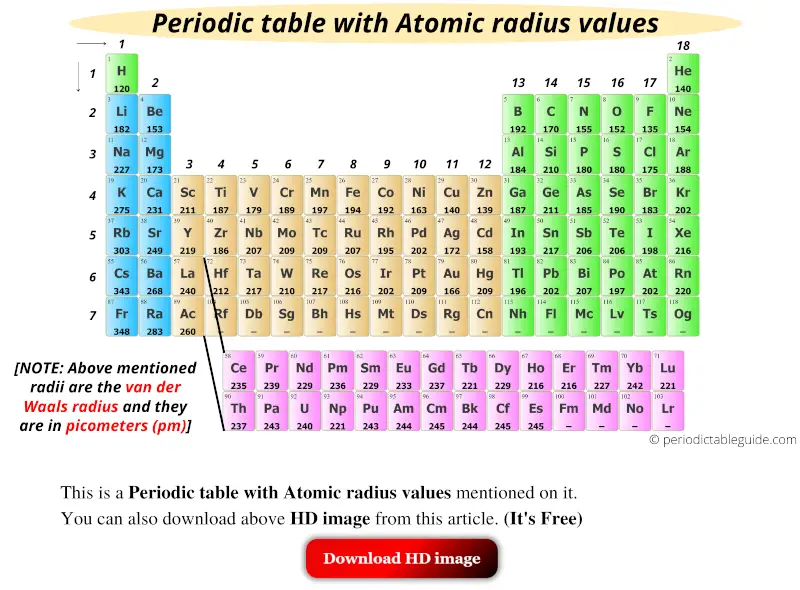
Get the Periodic table with Atomic radius values (Img+Chart)
How Is Atomic Size Measured Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic size is difficult to. Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. Atomic size is the distance from the nucleus to the edge of the electron cloud. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located.
From slideplayer.com
ATOMIC THEORY, PERIODIC CLASSIFICATION AND PROPERTIES OF ELEMENT ppt How Is Atomic Size Measured Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. Atomic size is difficult to. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. Have you ever wondered just how tiny atoms can be, or what determines their size? In basic chemistry, the atomic. How Is Atomic Size Measured.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy How Is Atomic Size Measured Atomic size is difficult to. Have you ever wondered just how tiny atoms can be, or what determines their size? The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure,. How Is Atomic Size Measured.
From www.flinnsci.ca
Atomic Sizes and Radii Charts for Chemistry How Is Atomic Size Measured The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. Atomic size is difficult to. The edge of the electron cloud is not well. How Is Atomic Size Measured.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID How Is Atomic Size Measured Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is difficult to. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic.. How Is Atomic Size Measured.
From www.slideserve.com
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID2417977 How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Atomic size is the distance from the nucleus to the edge of the electron cloud. In basic chemistry, the atomic. How Is Atomic Size Measured.
From www.zianet.com
Chemistry Unit Three Elements and Atoms Fundamental Matter How Is Atomic Size Measured Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is difficult to. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. Atomic size is the distance between the centre of the nucleus of an atom and its outermost. How Is Atomic Size Measured.
From www.slideserve.com
PPT Periodic Relationships Among the Elements PowerPoint Presentation How Is Atomic Size Measured Atomic size is difficult to. Atomic size is the distance from the nucleus to the edge of the electron cloud. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. A. How Is Atomic Size Measured.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts How Is Atomic Size Measured In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. Atomic size is difficult to. Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are. How Is Atomic Size Measured.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Atomic size is difficult to. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity. How Is Atomic Size Measured.
From courses.lumenlearning.com
Atomic Size Introduction to Chemistry How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. Atomic size is difficult to. Atomic size is the distance from the nucleus to. How Is Atomic Size Measured.
From www.youtube.com
Atomic Properties Lec 09\ definition of atomic size \ 10 CLASS \\NCERT How Is Atomic Size Measured The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. Atomic size is difficult to. A comparison of the dimensions of atoms or ions. How Is Atomic Size Measured.
From www.slideserve.com
PPT Atomic Size PowerPoint Presentation, free download ID6875591 How Is Atomic Size Measured The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. In basic chemistry, the atomic radius is defined as the shortest distance between the. How Is Atomic Size Measured.
From studylib.net
16 How does atomic size change within groups and across periods? How Is Atomic Size Measured Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear. How Is Atomic Size Measured.
From www.slideserve.com
PPT Atomic Structure and Periodicity PowerPoint Presentation, free How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Atomic size is difficult to. Atomic size is the distance from the nucleus. How Is Atomic Size Measured.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5897085 How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. The edge of the electron cloud is not well. How Is Atomic Size Measured.
From www.youtube.com
Size and Mass of Atoms YouTube How Is Atomic Size Measured The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Atomic size. How Is Atomic Size Measured.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID How Is Atomic Size Measured Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. Atomic size is the distance from the nucleus to the edge of the electron cloud. The measurement of atomic or ionic size will depend on a number. How Is Atomic Size Measured.
From www.chem.fsu.edu
Electron Configurations How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Atomic size is difficult to. Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell.. How Is Atomic Size Measured.
From users.highland.edu
Sizes of Atoms and Ions How Is Atomic Size Measured Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance from the nucleus to the edge of the electron cloud. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic. How Is Atomic Size Measured.
From www.slideserve.com
PPT Matter Think Pair Share PowerPoint Presentation, free How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Atomic size is difficult to. Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance from the nucleus to the edge of the electron cloud. The edge of the electron cloud is. How Is Atomic Size Measured.
From www.shutterstock.com
Illustration Chemistry Atomic Radius Measure Size Stock Vector (Royalty How Is Atomic Size Measured Atomic size is the distance from the nucleus to the edge of the electron cloud. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. The edge of the electron. How Is Atomic Size Measured.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID3170268 How Is Atomic Size Measured Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions. How Is Atomic Size Measured.
From www.periodictableprintable.com
Biggest Atomic Size In Periodic Table Periodic Table Printable How Is Atomic Size Measured The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. A comparison of the dimensions of atoms or ions that have the same number. How Is Atomic Size Measured.
From www.vrogue.co
Periodic Table Of The Elements Atomic Radius vrogue.co How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell. How Is Atomic Size Measured.
From www.slideserve.com
PPT Atomic Size PowerPoint Presentation, free download ID2643682 How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. Atomic size is difficult to. Atomic size is the distance from the nucleus. How Is Atomic Size Measured.
From slideplayer.com
Electrons and Periodicity ppt download How Is Atomic Size Measured Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Atomic size is difficult to. Atomic size is the distance from the nucleus to the edge of the electron cloud.. How Is Atomic Size Measured.
From www.slideserve.com
PPT PERIODIC TRENDS PowerPoint Presentation, free download ID5955497 How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. Have you ever wondered just how tiny atoms can be, or what determines their size? In basic chemistry, the atomic. How Is Atomic Size Measured.
From www.slideserve.com
PPT Chapter 7 Periodic Properties PowerPoint Presentation, free How Is Atomic Size Measured Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. Have you ever wondered just how tiny atoms can be, or what determines their size? Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. A comparison of the dimensions of atoms or ions. How Is Atomic Size Measured.
From www.slideserve.com
PPT Periodic Properties of Elements PowerPoint Presentation, free How Is Atomic Size Measured The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal structures. In basic chemistry, the atomic radius is defined as the shortest distance between the. How Is Atomic Size Measured.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2381541 How Is Atomic Size Measured A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in. How Is Atomic Size Measured.
From www.out-class.org
Atomic Size Explained! OutClass How Is Atomic Size Measured Have you ever wondered just how tiny atoms can be, or what determines their size? The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within regular crystal. How Is Atomic Size Measured.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) How Is Atomic Size Measured Atomic size is the distance from the nucleus to the edge of the electron cloud. In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an.. How Is Atomic Size Measured.
From www.teachoo.com
Define atomic size. Give its unit of measurement. In modern periodic How Is Atomic Size Measured Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. Atomic size is the distance from the nucleus to the edge of the electron cloud. A comparison of the dimensions of atoms or ions. How Is Atomic Size Measured.
From www.slideserve.com
PPT Atomic size PowerPoint Presentation, free download ID3704333 How Is Atomic Size Measured Atomic size is the distance from the nucleus to the edge of the electron cloud. Atomic size is difficult to. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between. In basic chemistry, the atomic radius is defined as the shortest. How Is Atomic Size Measured.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID How Is Atomic Size Measured The edge of the electron cloud is not well defined, so chemists use other definitions of atomic. The measurement of atomic or ionic size will depend on a number of factors, including the covalent character of bonding in any particular molecule, coordination number, physical state (liquid, solid, gas), the identity of nearby atoms/ions, variation in crystal structure, and distortions within. How Is Atomic Size Measured.