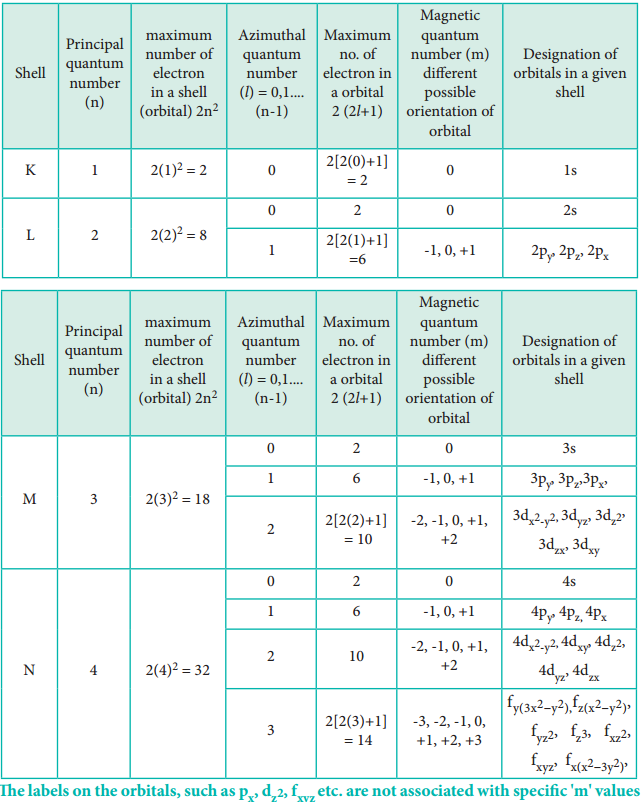
Can Ms Be 0 In Quantum Numbers . For example, if l = 0, then m l can be only 0; If n=1, l must be 0. There are four quantum numbers for atoms: Magnetic quantum number (m l) In atoms, there are a total of four quantum numbers: The allowed values of n are therefore 1, 2, 3, 4, and so on. If l = 1, m l can be −1, 0, or +1; The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. #n = 1, 2, 3,. The principal quantum number (n) cannot be zero. Quantum numbers can be used to describe the quantum state of an electron. When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. Orbitals that have same value of. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. And if l = 2, m l can be −2, −1, 0, +1, or +2.
from www.learninsta.com
If l = 1, m l can be −1, 0, or +1; And if l = 2, m l can be −2, −1, 0, +1, or +2. #n = 1, 2, 3,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The principal quantum number (n) cannot be zero. Quantum numbers can be used to describe the quantum state of an electron. Magnetic quantum number (m l) Orbitals that have same value of. If n=1, l must be 0. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles.
Quantum Numbers
Can Ms Be 0 In Quantum Numbers Orbitals that have same value of. Quantum numbers can be used to describe the quantum state of an electron. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. The allowed values of n are therefore 1, 2, 3, 4, and so on. And if l = 2, m l can be −2, −1, 0, +1, or +2. There are four quantum numbers for atoms: If l = 1, m l can be −1, 0, or +1; Orbitals that have same value of. Magnetic quantum number (m l) #n = 1, 2, 3,. The principal quantum number (n) cannot be zero. If n=1, l must be 0. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. In atoms, there are a total of four quantum numbers: For example, if l = 0, then m l can be only 0; When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4.
From www.youtube.com
Chemistry Lesson 11 Figuring Out the Quantum Numbers YouTube Can Ms Be 0 In Quantum Numbers And if l = 2, m l can be −2, −1, 0, +1, or +2. Orbitals that have same value of. The allowed values of n are therefore 1, 2, 3, 4, and so on. The principal quantum number (n) cannot be zero. If n=1, l must be 0. The principal quantum number (n), the orbital angular momentum quantum number. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 Can Ms Be 0 In Quantum Numbers If l = 1, m l can be −1, 0, or +1; #n = 1, 2, 3,. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. The principal quantum number (n) cannot be zero. In atoms, there are a total of four quantum numbers: Magnetic quantum number (m. Can Ms Be 0 In Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Can Ms Be 0 In Quantum Numbers If n=1, l must be 0. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Magnetic quantum number (m l) The principal quantum number (n) cannot be zero. Orbitals that have same value of. #n = 1, 2, 3,.. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Spin Quantum Number, m s PowerPoint Presentation, free download Can Ms Be 0 In Quantum Numbers Orbitals that have same value of. For example, if l = 0, then m l can be only 0; And if l = 2, m l can be −2, −1, 0, +1, or +2. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. #n = 1, 2, 3,. If. Can Ms Be 0 In Quantum Numbers.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog Can Ms Be 0 In Quantum Numbers If n=1, l must be 0. And if l = 2, m l can be −2, −1, 0, +1, or +2. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. Quantum numbers can be used to describe the quantum state of an electron. Orbitals that have same value. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 Can Ms Be 0 In Quantum Numbers The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The allowed values of n are therefore 1, 2, 3, 4, and so on. Magnetic quantum number (m l) #n = 1, 2, 3,. When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. In. Can Ms Be 0 In Quantum Numbers.
From www.youtube.com
IAS ( UPSC) Preparation Quantum numbers YouTube Can Ms Be 0 In Quantum Numbers If n=1, l must be 0. There are four quantum numbers for atoms: Orbitals that have same value of. In atoms, there are a total of four quantum numbers: When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. If l = 1, m l can be −1, 0, or +1; Magnetic quantum number (m. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a Can Ms Be 0 In Quantum Numbers If n=1, l must be 0. The allowed values of n are therefore 1, 2, 3, 4, and so on. Magnetic quantum number (m l) The principal quantum number (n) cannot be zero. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. In atoms, there are a total of. Can Ms Be 0 In Quantum Numbers.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Can Ms Be 0 In Quantum Numbers #n = 1, 2, 3,. In atoms, there are a total of four quantum numbers: The allowed values of n are therefore 1, 2, 3, 4, and so on. Orbitals that have same value of. There are four quantum numbers for atoms: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 Can Ms Be 0 In Quantum Numbers The principal quantum number (n) cannot be zero. And if l = 2, m l can be −2, −1, 0, +1, or +2. #n = 1, 2, 3,. Quantum numbers can be used to describe the quantum state of an electron. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and. Can Ms Be 0 In Quantum Numbers.
From www.learninsta.com
Quantum Numbers Can Ms Be 0 In Quantum Numbers The allowed values of n are therefore 1, 2, 3, 4, and so on. #n = 1, 2, 3,. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. If n=1, l must be 0. And if l = 2, m l can be −2, −1, 0, +1, or. Can Ms Be 0 In Quantum Numbers.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS Can Ms Be 0 In Quantum Numbers Magnetic quantum number (m l) For example, if l = 0, then m l can be only 0; There are four quantum numbers for atoms: If n=1, l must be 0. When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. The azimuthal quantum number is significant since it indicates the atomic orbital shape and. Can Ms Be 0 In Quantum Numbers.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash Can Ms Be 0 In Quantum Numbers If l = 1, m l can be −1, 0, or +1; If n=1, l must be 0. Quantum numbers can be used to describe the quantum state of an electron. The allowed values of n are therefore 1, 2, 3, 4, and so on. Magnetic quantum number (m l) #n = 1, 2, 3,. The principal quantum number (n),. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT QUANTUM MECHANICAL MODEL OF THE ATOM PowerPoint Presentation Can Ms Be 0 In Quantum Numbers In atoms, there are a total of four quantum numbers: #n = 1, 2, 3,. Magnetic quantum number (m l) Orbitals that have same value of. Quantum numbers can be used to describe the quantum state of an electron. For example, if l = 0, then m l can be only 0; When n = 2, l = 0 indicates. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download Can Ms Be 0 In Quantum Numbers For example, if l = 0, then m l can be only 0; When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. Orbitals that have same value of. #n = 1, 2, 3,. The allowed values of n are therefore 1, 2, 3, 4, and so on. And if l = 2, m l. Can Ms Be 0 In Quantum Numbers.
From www.youtube.com
Quantum Numbers Explained (A fully explained lesson) YouTube Can Ms Be 0 In Quantum Numbers When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. In atoms, there are a total of four quantum numbers: If l = 1, m l can be −1, 0, or +1; There are four quantum numbers for atoms: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 Can Ms Be 0 In Quantum Numbers There are four quantum numbers for atoms: Orbitals that have same value of. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. #n = 1, 2, 3,. And if l = 2, m l can be −2, −1, 0, +1, or +2. If n=1, l must be 0.. Can Ms Be 0 In Quantum Numbers.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Can Ms Be 0 In Quantum Numbers The principal quantum number (n) cannot be zero. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Orbitals that have same value of. The allowed values of n are therefore 1, 2, 3, 4, and so on. When n = 2, l = 0 indicates 2s2 and l =. Can Ms Be 0 In Quantum Numbers.
From slideplayer.com
Quantum Numbers. ppt download Can Ms Be 0 In Quantum Numbers Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. If l = 1, m l can be −1, 0, or +1; #n = 1, 2, 3,. The principal quantum number (n) cannot be zero. Magnetic quantum. Can Ms Be 0 In Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Can Ms Be 0 In Quantum Numbers And if l = 2, m l can be −2, −1, 0, +1, or +2. In atoms, there are a total of four quantum numbers: The principal quantum number (n) cannot be zero. If n=1, l must be 0. Quantum numbers can be used to describe the quantum state of an electron. Orbitals that have same value of. When n. Can Ms Be 0 In Quantum Numbers.
From quantum-lesson.blogspot.com
How To Find 4 Quantum Numbers Of An Element Can Ms Be 0 In Quantum Numbers The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. The principal quantum number (n) cannot be zero. In atoms, there are a total of four quantum numbers: And if l = 2, m l can be −2, −1, 0, +1, or +2. For example, if l = 0, then. Can Ms Be 0 In Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Can Ms Be 0 In Quantum Numbers Orbitals that have same value of. If n=1, l must be 0. Quantum numbers can be used to describe the quantum state of an electron. If l = 1, m l can be −1, 0, or +1; There are four quantum numbers for atoms: #n = 1, 2, 3,. For example, if l = 0, then m l can be. Can Ms Be 0 In Quantum Numbers.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan Can Ms Be 0 In Quantum Numbers The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. The allowed values of n are therefore 1, 2, 3, 4, and so on. #n = 1, 2, 3,. The principal quantum number (n) cannot be zero. If n=1, l must be 0. If l = 1, m l. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation Can Ms Be 0 In Quantum Numbers The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. Orbitals that have same value of. #n = 1, 2, 3,. The principal quantum number (n) cannot be zero. For example, if l = 0, then m l can be only 0; If l = 1, m l can. Can Ms Be 0 In Quantum Numbers.
From slidetodoc.com
Quantum Numbers Activity Quantum Numbers Quantum numbers tell Can Ms Be 0 In Quantum Numbers In atoms, there are a total of four quantum numbers: And if l = 2, m l can be −2, −1, 0, +1, or +2. If n=1, l must be 0. There are four quantum numbers for atoms: Magnetic quantum number (m l) The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml),. Can Ms Be 0 In Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Can Ms Be 0 In Quantum Numbers Orbitals that have same value of. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. Magnetic quantum number (m l) When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. And if l = 2, m l can be −2, −1, 0, +1,. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Chemistry I HonorsUnit 3 Quantum Mechanical Model of the Atom Can Ms Be 0 In Quantum Numbers The principal quantum number (n) cannot be zero. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. In atoms, there are a total of four quantum numbers: When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. The allowed values of n are. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 Can Ms Be 0 In Quantum Numbers The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. If n=1, l must be 0. #n = 1, 2, 3,. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. There are four quantum numbers for atoms: And. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum numbers PowerPoint Presentation, free download ID9387825 Can Ms Be 0 In Quantum Numbers Quantum numbers can be used to describe the quantum state of an electron. If l = 1, m l can be −1, 0, or +1; The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles. The principal quantum number (n) cannot be zero. The principal quantum number (n), the. Can Ms Be 0 In Quantum Numbers.
From www.numerade.com
Which of the following set of quantum numbers (ordered n, € , ml, ms Can Ms Be 0 In Quantum Numbers The principal quantum number (n) cannot be zero. Quantum numbers can be used to describe the quantum state of an electron. If n=1, l must be 0. In atoms, there are a total of four quantum numbers: When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. And if l = 2, m l can. Can Ms Be 0 In Quantum Numbers.
From studylib.net
Quantum Numbers Can Ms Be 0 In Quantum Numbers There are four quantum numbers for atoms: In atoms, there are a total of four quantum numbers: If n=1, l must be 0. Magnetic quantum number (m l) And if l = 2, m l can be −2, −1, 0, +1, or +2. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml),. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Lecture 7 Quantum Numbers and Electron Configurations PowerPoint Can Ms Be 0 In Quantum Numbers Orbitals that have same value of. If l = 1, m l can be −1, 0, or +1; Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n) cannot be zero. The azimuthal quantum number is significant since it indicates the atomic orbital shape and vibrantly affects chemical bonds and bond angles.. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download Can Ms Be 0 In Quantum Numbers In atoms, there are a total of four quantum numbers: The principal quantum number (n) cannot be zero. Orbitals that have same value of. When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron. Magnetic. Can Ms Be 0 In Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Can Ms Be 0 In Quantum Numbers When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. If l = 1, m l can be −1, 0, or +1; In atoms, there are a total of four quantum numbers: And if l = 2, m l can be −2, −1, 0, +1, or +2. The azimuthal quantum number is significant since it. Can Ms Be 0 In Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 Can Ms Be 0 In Quantum Numbers For example, if l = 0, then m l can be only 0; The principal quantum number (n) cannot be zero. When n = 2, l = 0 indicates 2s2 and l = 1 indicates 2p4. Quantum numbers can be used to describe the quantum state of an electron. Orbitals that have same value of. #n = 1, 2, 3,.. Can Ms Be 0 In Quantum Numbers.