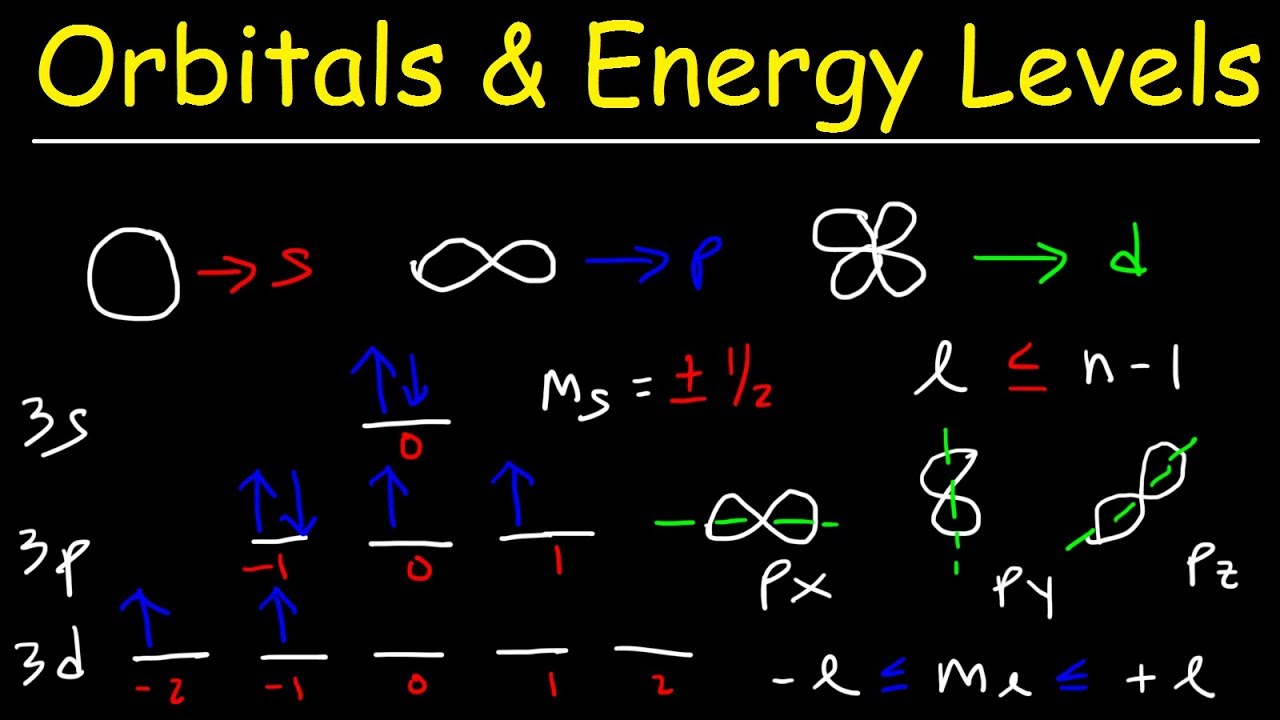
How Many Energy Levels Are There In An Atom . Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy level, like each electron shell’s own energy level. There are at most two electrons per orbital. These levels (also known as electron shells) are fixed. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The arrangement of electrons in an atom is called the electronic configuration.
from www.youtube.com
Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. The energy expressed by an electron in a substance’s band model is referred to as energy levels. These levels (also known as electron shells) are fixed. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy level, like each electron shell’s own energy level. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. There are at most two electrons per orbital. The arrangement of electrons in an atom is called the electronic configuration.
Orbitals, Atomic Energy Levels, & Sublevels Explained Basic
How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The maximum number of electrons at a given energy level depends on its number of orbitals. There are at most two electrons per orbital. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. These levels (also known as electron shells) are fixed. Each subshell has a different energy level, like each electron shell’s own energy level. The arrangement of electrons in an atom is called the electronic configuration.
From www.slideserve.com
PPT Chapter 71 Electrons and their Energy Levels Part 2 PowerPoint How Many Energy Levels Are There In An Atom The arrangement of electrons in an atom is called the electronic configuration. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. There are at most two electrons per orbital. The maximum number of electrons at a given energy level. How Many Energy Levels Are There In An Atom.
From www.alamy.com
Radium atom, with mass and energy levels. Vector illustration Stock How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. There are at most two electrons per orbital. These levels (also known as electron shells) are fixed. The arrangement. How Many Energy Levels Are There In An Atom.
From pijaeducation.com
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. There are at most two electrons per orbital. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy level, like each electron shell’s. How Many Energy Levels Are There In An Atom.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Many Energy Levels Are There In An Atom There are at most two electrons per orbital. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT The BohrRutherford Atom PowerPoint Presentation, free download How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy level, like each electron shell’s own energy level. The energy expressed by an electron in a substance’s band model is referred to as energy levels. There are at most. How Many Energy Levels Are There In An Atom.
From www.online-sciences.com
Atomic structure of matter, Energy levels, Electronic distribution and How Many Energy Levels Are There In An Atom Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. The arrangement of electrons in an atom is called the electronic configuration. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the. How Many Energy Levels Are There In An Atom.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. Each subshell has a different energy level, like each electron shell’s own energy level. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on. How Many Energy Levels Are There In An Atom.
From kdi-ppi.com
Exploring Energy Level Diagram Examples From Atoms to Molecules How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. The energy expressed by an electron in a substance’s band model is referred to as energy levels. These levels (also known as electron shells) are fixed. There are at most two electrons per orbital. The arrangement of electrons in an atom is called the electronic configuration.. How Many Energy Levels Are There In An Atom.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 How Many Energy Levels Are There In An Atom The maximum number of electrons at a given energy level depends on its number of orbitals. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. The arrangement of electrons in an atom is called the electronic configuration. These levels. How Many Energy Levels Are There In An Atom.
From scientifictutor.org
Chem Energy Levels Scientific Tutor How Many Energy Levels Are There In An Atom Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. The arrangement of electrons in an atom is called the electronic configuration. The energy expressed by an electron in a substance’s band model is referred to as energy levels. Each. How Many Energy Levels Are There In An Atom.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table How Many Energy Levels Are There In An Atom The maximum number of electrons at a given energy level depends on its number of orbitals. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The arrangement of electrons in an atom is called the electronic configuration. There are at most two electrons per. How Many Energy Levels Are There In An Atom.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy How Many Energy Levels Are There In An Atom The arrangement of electrons in an atom is called the electronic configuration. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Each subshell has a different energy level, like each electron shell’s own energy level. Each element has a unique set of energy levels,. How Many Energy Levels Are There In An Atom.
From www.youtube.com
Chemistry Basics Atom Structure, Energy Levels, Sublevels, Orbitals How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. The energy expressed by an electron in a substance’s band model is referred to as energy levels. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. There are at most. How Many Energy Levels Are There In An Atom.
From www.stonecoldhands.com
Electron Shells and Orbitals Stone Cold Chemistry Talk How Many Energy Levels Are There In An Atom The arrangement of electrons in an atom is called the electronic configuration. There are at most two electrons per orbital. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. Each subshell has a different energy level, like each electron. How Many Energy Levels Are There In An Atom.
From www.numerade.com
this energy diagram shows the allowed energy levels of an electron in a How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. The energy expressed by an electron in a substance’s band model is referred to as energy levels. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT The Modern Quantum Atom PowerPoint Presentation ID6689669 How Many Energy Levels Are There In An Atom Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. There are at most two electrons per orbital. The arrangement of electrons in an atom is called the electronic configuration. In this explainer, we will learn how to describe and. How Many Energy Levels Are There In An Atom.
From mavink.com
Periodic Table With Energy Levels How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Each subshell has a different energy level, like each electron shell’s own energy level. The arrangement of electrons in an atom is called the electronic configuration. Each element has a unique set of energy levels,. How Many Energy Levels Are There In An Atom.
From scientifictutor.org
Chem Energy Levels Scientific Tutor How Many Energy Levels Are There In An Atom The maximum number of electrons at a given energy level depends on its number of orbitals. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The energy expressed by an electron in a substance’s band model is referred to as energy levels. There are. How Many Energy Levels Are There In An Atom.
From www.shutterstock.com
Energy Levels Of The Atom Over 1,974 RoyaltyFree Licensable Stock How Many Energy Levels Are There In An Atom The maximum number of electrons at a given energy level depends on its number of orbitals. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. Each subshell has a different energy level, like each electron shell’s own energy level.. How Many Energy Levels Are There In An Atom.
From wiringguidefrosts.z19.web.core.windows.net
Electron Orbital Diagram Chart How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. Each subshell has a different. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT Chapters 45 The Atom PowerPoint Presentation, free download How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. The energy expressed by an electron in a substance’s band model is referred to as energy levels. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. There are at most. How Many Energy Levels Are There In An Atom.
From cronodon.com
Introduction to Atoms How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. The arrangement of electrons in an atom is called the electronic configuration. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The maximum number of electrons at a given energy level depends on its number of orbitals. There. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT ATOMIC THEORY REVIEW PowerPoint Presentation, free download ID How Many Energy Levels Are There In An Atom The energy expressed by an electron in a substance’s band model is referred to as energy levels. Each subshell has a different energy level, like each electron shell’s own energy level. There are at most two electrons per orbital. The maximum number of electrons at a given energy level depends on its number of orbitals. The arrangement of electrons in. How Many Energy Levels Are There In An Atom.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. The energy expressed by an. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT Ch 5 Atomic Structure and the Periodic Table PowerPoint How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy level, like each electron shell’s own energy level. There are at most two. How Many Energy Levels Are There In An Atom.
From hubpages.com
Atoms and Atomic Structure How Many Energy Levels Are There In An Atom The energy expressed by an electron in a substance’s band model is referred to as energy levels. The arrangement of electrons in an atom is called the electronic configuration. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. Each. How Many Energy Levels Are There In An Atom.
From www.schoolphysics.co.uk
schoolphysics How Many Energy Levels Are There In An Atom The energy expressed by an electron in a substance’s band model is referred to as energy levels. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. There are. How Many Energy Levels Are There In An Atom.
From mavink.com
Niels Bohr Atomic Model How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. The energy expressed by an electron in a substance’s band. How Many Energy Levels Are There In An Atom.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. The maximum number of electrons at a given energy level depends on its number of orbitals. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. There are at most two electrons per orbital. Each element. How Many Energy Levels Are There In An Atom.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube How Many Energy Levels Are There In An Atom Each subshell has a different energy level, like each electron shell’s own energy level. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. There are at most two electrons per orbital. Each element has a unique set of energy levels, and so the frequencies. How Many Energy Levels Are There In An Atom.
From www.youtube.com
Energy levels and sub energy levels of atom. YouTube How Many Energy Levels Are There In An Atom The energy expressed by an electron in a substance’s band model is referred to as energy levels. These levels (also known as electron shells) are fixed. The arrangement of electrons in an atom is called the electronic configuration. Each subshell has a different energy level, like each electron shell’s own energy level. Each element has a unique set of energy. How Many Energy Levels Are There In An Atom.
From www.thoughtco.com
Electron Configuration Chart How Many Energy Levels Are There In An Atom In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. The maximum number of electrons at a given energy level depends on its number of orbitals. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The arrangement. How Many Energy Levels Are There In An Atom.
From www.slideserve.com
PPT Properties of Atoms and the Periodic Table PowerPoint How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The maximum number of electrons at a given energy level depends on its number of orbitals. There are at most two electrons per orbital. In this explainer, we will learn how to describe and. How Many Energy Levels Are There In An Atom.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons How Many Energy Levels Are There In An Atom There are at most two electrons per orbital. Each subshell has a different energy level, like each electron shell’s own energy level. These levels (also known as electron shells) are fixed. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular. How Many Energy Levels Are There In An Atom.
From www.youtube.com
Orbitals, Atomic Energy Levels, & Sublevels Explained Basic How Many Energy Levels Are There In An Atom These levels (also known as electron shells) are fixed. The energy expressed by an electron in a substance’s band model is referred to as energy levels. The arrangement of electrons in an atom is called the electronic configuration. The maximum number of electrons at a given energy level depends on its number of orbitals. Each subshell has a different energy. How Many Energy Levels Are There In An Atom.