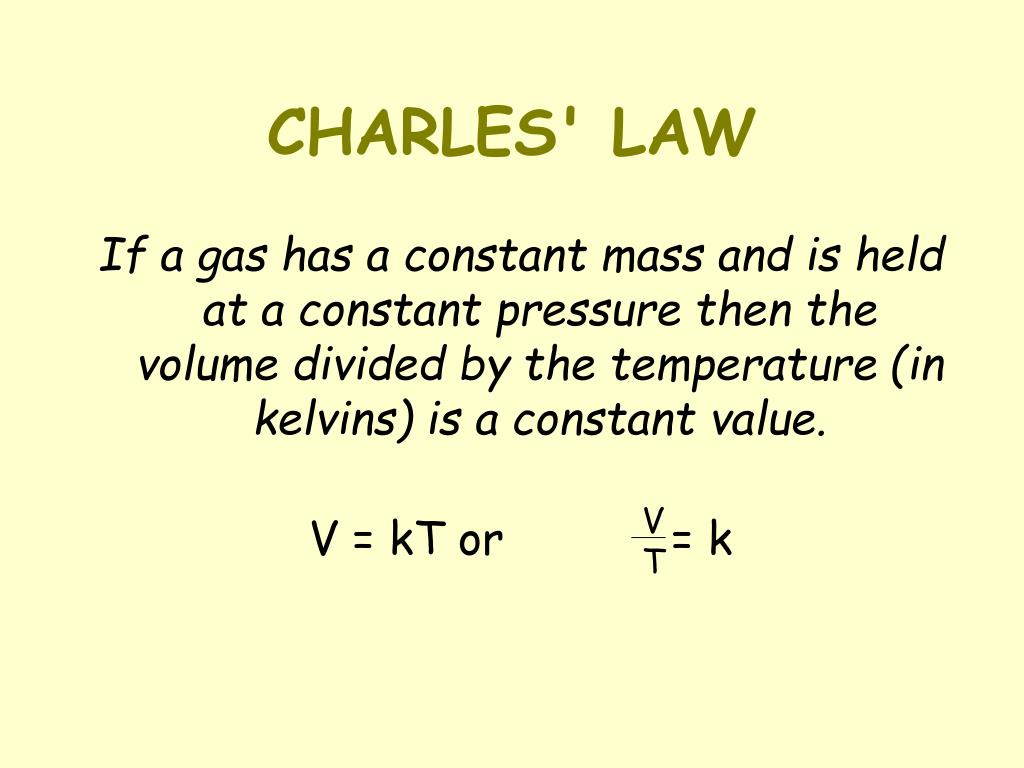
How To Keep Pressure Constant In Charles Law . The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. This is to keep the pressure constant at atmospheric. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. The capillary tube should have one open end at the top and a closed end at the bottom. At constant pressure, the volume of a fixed amount of gas is directly proportional to its.
from www.slideserve.com
In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The capillary tube should have one open end at the top and a closed end at the bottom. This is to keep the pressure constant at atmospheric. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed.
PPT Charles's Law PowerPoint Presentation, free download ID5773078
How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. The capillary tube should have one open end at the top and a closed end at the bottom. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. This is to keep the pressure constant at atmospheric. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant.
From www.youtube.com
Charles's Law Solving for Final Temperature YouTube How To Keep Pressure Constant In Charles Law Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles's law states that the volume of a given mass of gas varies directly with. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Charles’ Law PowerPoint Presentation, free download ID3180582 How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. The capillary tube should have one open end at the top and a closed end at the bottom. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles’s law states that if a given quantity of. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Charles’ Law PowerPoint Presentation, free download ID5759545 How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. In practise, one could adjust the temperature, wait for. How To Keep Pressure Constant In Charles Law.
From www.expii.com
Charles's Law — Overview & Formula Expii How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. In practise, one could adjust. How To Keep Pressure Constant In Charles Law.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing How To Keep Pressure Constant In Charles Law Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT The Behavior of Gases PowerPoint Presentation, free download ID How To Keep Pressure Constant In Charles Law This is to keep the pressure constant at atmospheric. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles's law states that the volume of. How To Keep Pressure Constant In Charles Law.
From www.diplomageeks.com
Boyles law and Charles law its validity How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust. How To Keep Pressure Constant In Charles Law.
From general.chemistrysteps.com
Charle’s Law Chemistry Steps How To Keep Pressure Constant In Charles Law The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. This is to keep the pressure constant at atmospheric. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas,. How To Keep Pressure Constant In Charles Law.
From www.chemistrylearner.com
Charles’ Law Statement, Formula, Examples, and Graph How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The capillary tube should have one open end at the top and a closed end at the bottom. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t. How To Keep Pressure Constant In Charles Law.
From www.visionlearning.org
Properties of Gases Chemistry Visionlearning How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. At constant pressure, the volume of a fixed amount. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Charles's Law PowerPoint Presentation, free download ID5773078 How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. The capillary tube should have one open end at the top and a closed end at the bottom. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. Charles's law. How To Keep Pressure Constant In Charles Law.
From chemistryguru.com.sg
Ideal Gas Law and Applications How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. The equation for charles's law can be expressed as v 1 /t 1 =v 2. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2963944 How To Keep Pressure Constant In Charles Law Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. In this explainer,. How To Keep Pressure Constant In Charles Law.
From physicsteacher.in
Gas laws Statement, Formula, graph Charles’ law, Boyle's & Pressure law How To Keep Pressure Constant In Charles Law The capillary tube should have one open end at the top and a closed end at the bottom. This is to keep the pressure constant at atmospheric. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. In this explainer, we will learn how to. How To Keep Pressure Constant In Charles Law.
From slideplayer.com
Chapter 10 Gases Their Properties and Behavior ppt download How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are. How To Keep Pressure Constant In Charles Law.
From www.sliderbase.com
Gas Temperature Volume and Pressure Presentation Physics How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The capillary tube should have one open end at the top and a closed end at the bottom. This is to keep the pressure constant at atmospheric. At constant pressure, the volume of a. How To Keep Pressure Constant In Charles Law.
From www.sliderbase.com
Gas Laws Presentation Chemistry How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. The capillary tube should have one open end at the top and a closed end at the bottom. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component. How To Keep Pressure Constant In Charles Law.
From www.pinterest.com
Charles's Law Definition, Formula, Examples Charles law, Teaching How To Keep Pressure Constant In Charles Law The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles’s law states that if a given quantity of gas is held at a constant pressure, its. How To Keep Pressure Constant In Charles Law.
From www.chegg.com
Solved Question 8 Charles's law states that if the pressure How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. This is to keep the pressure constant at atmospheric. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles’s law states that if a given quantity. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. The equation for charles's law can be expressed. How To Keep Pressure Constant In Charles Law.
From slideplayer.com
Gas Laws. ppt download How To Keep Pressure Constant In Charles Law Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles’s law. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint How To Keep Pressure Constant In Charles Law Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to.. How To Keep Pressure Constant In Charles Law.
From www.nagwa.com
Question Video Finding the Initial Temperature of a Gas Using Charles How To Keep Pressure Constant In Charles Law Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In practise, one could adjust the temperature, wait for the. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Boyle’s Law and Charles’ Law PowerPoint Presentation, free How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. This is to keep the pressure constant at atmospheric. The equation for charles's law can be expressed. How To Keep Pressure Constant In Charles Law.
From solvedlib.com
Use Charles's law to complete table (assume pres… SolvedLib How To Keep Pressure Constant In Charles Law The capillary tube should have one open end at the top and a closed end at the bottom. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. This is to keep the pressure constant at atmospheric.. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT The Gas Laws PowerPoint Presentation, free download ID4135634 How To Keep Pressure Constant In Charles Law Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. This is to keep the pressure constant at atmospheric. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint How To Keep Pressure Constant In Charles Law In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust. How To Keep Pressure Constant In Charles Law.
From www.slideserve.com
PPT Charles’ Law PowerPoint Presentation, free download ID5759545 How To Keep Pressure Constant In Charles Law Charles's law describes the directly proportional relationship between the volume and temperature (in kelvin) of a fixed. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present. How To Keep Pressure Constant In Charles Law.
From stock.adobe.com
Charles Law Infographic Diagram Example helium balloon when volume How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. At constant pressure, the volume of a. How To Keep Pressure Constant In Charles Law.
From study.com
How to Write Pressure Equilibrium Constant Expressions Chemistry How To Keep Pressure Constant In Charles Law This is to keep the pressure constant at atmospheric. At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles's law states that the volume of a given. How To Keep Pressure Constant In Charles Law.
From owlcation.com
The Theories and Behavior of Gas Owlcation How To Keep Pressure Constant In Charles Law The capillary tube should have one open end at the top and a closed end at the bottom. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept. Charles’s law states that if a given quantity of gas is held at a constant pressure, its. How To Keep Pressure Constant In Charles Law.
From www.nagwa.com
Lesson Video Charles’ Law Nagwa How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. The equation for charles's. How To Keep Pressure Constant In Charles Law.
From askwillonline.com
Chapter 13 and 14 Physics A2 OCR Revision Ask Will Online How To Keep Pressure Constant In Charles Law In practise, one could adjust the temperature, wait for the volume to settle down, and then adjust the fixed weight, compensating for changes the atmospheric component to. Charles’s law states that if a given quantity of gas is held at a constant pressure, its volume is directly proportional to the absolute. Charles's law states that the volume of a given. How To Keep Pressure Constant In Charles Law.
From www.chegg.com
Solved Constants Periodic Table Charles's law states that How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas,. How To Keep Pressure Constant In Charles Law.
From medium.com
Charles law volume and temperature Teach Chemistry How To Keep Pressure Constant In Charles Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its. In this explainer, we will learn how to use the formula 𝑉 𝑇 = constant (charles’ law) to calculate the volume or temperature of a gas. The capillary tube should have one open end at the top and a closed end at the bottom.. How To Keep Pressure Constant In Charles Law.