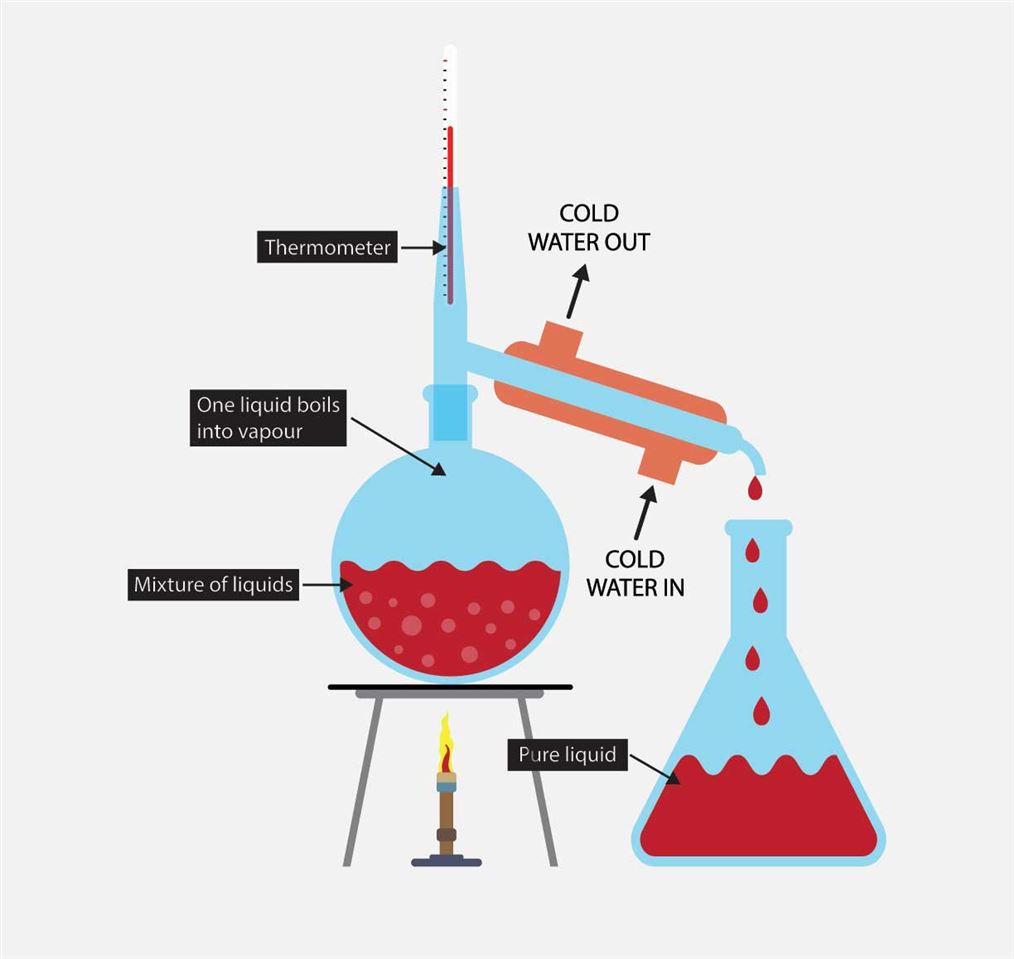
Vacuum Distillation Examples . An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. How does vacuum distillation work? The heat exchanger comprises a bundle. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum.
from www.peoplesbourbonreview.com
An approximate distillation temperature can be calculated using the known boiling point of the compound (at. The heat exchanger comprises a bundle. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. How does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is particularly useful for distilling products of boiling points over 100oc.
What is Distillation and How is Liquor Made? The People's Bourbon Review
Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. The heat exchanger comprises a bundle. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. How does vacuum distillation work? An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or.
From www.thoughtco.com
What Is Distillation? Principles and Uses Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. This is particularly useful for distilling products of boiling points over 100oc. The heat exchanger comprises a bundle. An approximate distillation temperature can be calculated using. Vacuum Distillation Examples.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. The heat exchanger comprises a bundle. Vacuum distillation is a technique for the purification or separation. Vacuum Distillation Examples.
From www.youtube.com
Solvent Vacuum Distillation YouTube Vacuum Distillation Examples In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50,. Vacuum Distillation Examples.
From blog.digivac.com
3 Benefits of Vacuum Distillation in Low and Medium Vacuum Vacuum Distillation Examples Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. How does vacuum distillation work? The heat exchanger comprises a bundle. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the. Vacuum Distillation Examples.
From www.researchgate.net
The schematic for the vacuum distillation process. Download Vacuum Distillation Examples How does vacuum distillation work? In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. The heat exchanger comprises a bundle. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling. Vacuum Distillation Examples.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Vacuum Distillation Examples Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. The heat exchanger comprises a bundle. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the. Vacuum Distillation Examples.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Vacuum Distillation Examples.
From www.youtube.com
How To Draw Simple Distillation Diagram step by step YouTube Vacuum Distillation Examples Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. The heat exchanger comprises a bundle. How does vacuum distillation work? An approximate distillation temperature. Vacuum Distillation Examples.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Vacuum Distillation Examples In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This. Vacuum Distillation Examples.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Vacuum Distillation Examples How does vacuum distillation work? Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. The heat exchanger comprises a bundle. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a technique. Vacuum Distillation Examples.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes Vacuum Distillation Examples Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken. Vacuum Distillation Examples.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific Vacuum Distillation Examples In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Examples.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Vacuum Distillation Examples The heat exchanger comprises a bundle. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. How does vacuum distillation work? In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling. Vacuum Distillation Examples.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Vacuum Distillation Examples How does vacuum distillation work? An approximate distillation temperature can be calculated using the known boiling point of the compound (at. This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. The heat exchanger comprises a bundle. Vacuum. Vacuum Distillation Examples.
From www.baronusa.com
Vacuum Distillation Units — BaronUSA Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. The heat exchanger comprises a bundle. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. How does vacuum distillation work? In the. Vacuum Distillation Examples.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download Vacuum Distillation Examples An approximate distillation temperature can be calculated using the known boiling point of the compound (at. How does vacuum distillation work? The heat exchanger comprises a bundle. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. In the vacuum distillation process,. Vacuum Distillation Examples.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Vacuum Distillation Examples An approximate distillation temperature can be calculated using the known boiling point of the compound (at. This is particularly useful for distilling products of boiling points over 100oc. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In. Vacuum Distillation Examples.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Vacuum Distillation Examples The heat exchanger comprises a bundle. This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a distillation carried. Vacuum Distillation Examples.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Vacuum Distillation Examples How does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. An approximate distillation temperature can be calculated using the known boiling point of. Vacuum Distillation Examples.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Vacuum Distillation Examples A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. An approximate distillation temperature. Vacuum Distillation Examples.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery The Petro Solutions Vacuum Distillation Examples Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. How does vacuum distillation work? This is particularly useful for distilling products of boiling points over 100oc. The heat exchanger comprises a bundle. Vacuum distillation is a technique for. Vacuum Distillation Examples.
From glossary.periodni.com
Distillation Chemistry Dictionary & Glossary Vacuum Distillation Examples Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. How does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. An approximate distillation temperature can be calculated using the known boiling point of the compound (at.. Vacuum Distillation Examples.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Vacuum Distillation Examples Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. How does vacuum distillation work? A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under. Vacuum Distillation Examples.
From chemicalengineeringworld.com
vacuum distillation example Archives Chemical Engineering World Vacuum Distillation Examples A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. This is particularly useful for distilling products of. Vacuum Distillation Examples.
From www.e-education.psu.edu
Atmospheric and Vacuum Distillation Units FSC 432 Petroleum Refining Vacuum Distillation Examples The heat exchanger comprises a bundle. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. How does vacuum distillation work? An approximate distillation temperature can be calculated using the known boiling point of the compound (at. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is. Vacuum Distillation Examples.
From www.youtube.com
What is vacuum distillation use of vacuum distillation what is air Vacuum Distillation Examples In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. The heat exchanger comprises a bundle. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. How does vacuum distillation work? An approximate distillation temperature can be calculated using the known boiling point of the compound. Vacuum Distillation Examples.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Vacuum Distillation Examples Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. How does vacuum distillation work? Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. The heat exchanger comprises. Vacuum Distillation Examples.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Vacuum Distillation Examples This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. The heat exchanger comprises a bundle. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or.. Vacuum Distillation Examples.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Vacuum Distillation Examples In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. This is particularly useful for distilling products of boiling points over 100oc. How does vacuum distillation work? Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown. Vacuum Distillation Examples.
From www.researchgate.net
Vacuum distillation apparatus. Download Scientific Diagram Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The heat exchanger comprises a bundle. How does vacuum distillation work? An approximate distillation temperature can be calculated using the known boiling point of the compound (at. A vacuum. Vacuum Distillation Examples.
From www.youtube.com
Vacuum Distillation YouTube Vacuum Distillation Examples A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. How does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is particularly useful for distilling products of boiling points over 100oc. An approximate distillation temperature can be calculated. Vacuum Distillation Examples.
From chemicaltweak.com
When Vacuum Distillation Is Selected Application And Uses Of VDU Vacuum Distillation Examples Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. An approximate distillation temperature can be calculated using the known boiling point of the compound (at. This is particularly useful for distilling products of boiling points over 100oc. How does vacuum distillation work? A vacuum distillation apparatus is. Vacuum Distillation Examples.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150. Vacuum Distillation Examples.
From www.youtube.com
Distillation under reduced pressure Vacuum Distillation Vacuum Distillation Examples Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of. Vacuum Distillation Examples.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Vacuum Distillation Examples This is particularly useful for distilling products of boiling points over 100oc. In the vacuum distillation process, the industrial wastewater is taken care of into a heat exchanger and evaporated under vacuum. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.. Vacuum Distillation Examples.