Equilibrium Constant Vs Equilibrium Expression . the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium.
from albanydexter.blogspot.com
equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the.
43+ equilibrium constant expression calculator AlbanyDexter
Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium.
From www.slideserve.com
PPT SAMPLE EXERCISE 15.1 Writing EquilibriumConstant Expressions Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. . Equilibrium Constant Vs Equilibrium Expression.
From lessonlibundertoned.z22.web.core.windows.net
Equilibrium Constant Expression Worksheet Equilibrium Constant Vs Equilibrium Expression the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT The Equilibrium Condition, the Equilibrium Constant and Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT CHAPTER 16 PowerPoint Presentation, free download ID4529646 Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. . Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
How to Write Equilibrium Constant Expressions Kc Keq Kp Basics Equilibrium Constant Vs Equilibrium Expression comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. . Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
Lecture 6 Equilibrium Constant Expression YouTube Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7. Equilibrium Constant Vs Equilibrium Expression.
From www.chegg.com
Solved Write the equilibrium constant expression for the Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of. Equilibrium Constant Vs Equilibrium Expression.
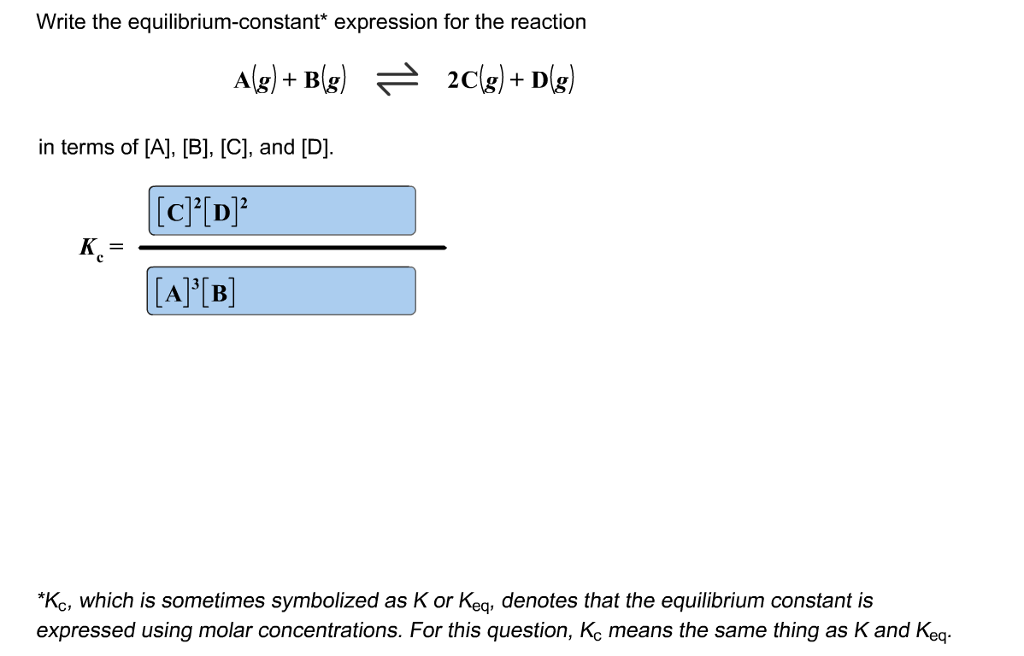
From oneclass.com
OneClass Write the equilibriumconstant* expression for the reaction A Equilibrium Constant Vs Equilibrium Expression comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Introduction to Chemical Equilibrium PowerPoint Presentation Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction. Equilibrium Constant Vs Equilibrium Expression.
From www.vrogue.co
Equilibrium Constant Definition And Expression Biolog vrogue.co Equilibrium Constant Vs Equilibrium Expression the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. write an equilibrium constant expression, such as k c, in terms of reactants and products and. Equilibrium Constant Vs Equilibrium Expression.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant, k, expresses the relationship between products and. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT The Equilibrium Constant Expression PowerPoint Presentation, free Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b,. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. . Equilibrium Constant Vs Equilibrium Expression.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Equilibrium Constant Vs Equilibrium Expression comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. write an equilibrium constant expression, such as k c, in. Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium. Equilibrium Constant Vs Equilibrium Expression.
From sharedocnow.blogspot.com
Pressure Equilibrium Constant Expression sharedoc Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT The Equilibrium Constant Expression PowerPoint Presentation, free Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. comparison of the data plots in figure 13.5 shows that both experimental. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT The Equilibrium Constant Expression PowerPoint Presentation, free Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. . Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium USING THE EQUILIBRIUM CONSTANT Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. write an equilibrium constant. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and. Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
Importance of Equilibrium Constant in Chemistry How do you determine Equilibrium Constant Vs Equilibrium Expression comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.4 is called the equilibrium equation, and the right. Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
7.2.1 Deduce the equilibrium constant expression (Kc) from the equation Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT SAMPLE EXERCISE 15.1 Writing EquilibriumConstant Expressions Equilibrium Constant Vs Equilibrium Expression the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2224637 Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant, k, expresses the relationship between products and. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Year 12 Chemistry PowerPoint Presentation, free download ID1931303 Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. write an equilibrium constant expression, such as k c, in terms of reactants and. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. . Equilibrium Constant Vs Equilibrium Expression.
From www.youtube.com
How to Write Equilibrium Constant Expression Kc and Kp Expression Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. comparison of the data. Equilibrium Constant Vs Equilibrium Expression.
From albanydexter.blogspot.com
43+ equilibrium constant expression calculator AlbanyDexter Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for. Equilibrium Constant Vs Equilibrium Expression.
From www.coursehero.com
[Solved] has the formulation equilibrium constant expression. Nz(q Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the. Equilibrium Constant Vs Equilibrium Expression.
From study.com
How to Write Equilibrium Expressions for Heterogeneous Reactions Equilibrium Constant Vs Equilibrium Expression the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and. Equilibrium Constant Vs Equilibrium Expression.
From en.differbetween.com
Difference Between Reaction Quotient and Equilibrium Constant Equilibrium Constant Vs Equilibrium Expression comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of. Equilibrium Constant Vs Equilibrium Expression.
From study.com
How to Write a Concentration Equilibrium Constant Expression Equilibrium Constant Vs Equilibrium Expression equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b,. Equilibrium Constant Vs Equilibrium Expression.
From www.slideserve.com
PPT Topics in CHM 1046 PowerPoint Presentation, free download ID Equilibrium Constant Vs Equilibrium Expression equation 15.2.4 is called the equilibrium equation, and the right side of equation 15.2.5 is called the equilibrium. write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium. . Equilibrium Constant Vs Equilibrium Expression.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Equilibrium Constant Vs Equilibrium Expression write an equilibrium constant expression, such as k c, in terms of reactants and products and the stoichiometry of the. comparison of the data plots in figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective. Equilibrium Constant Vs Equilibrium Expression.