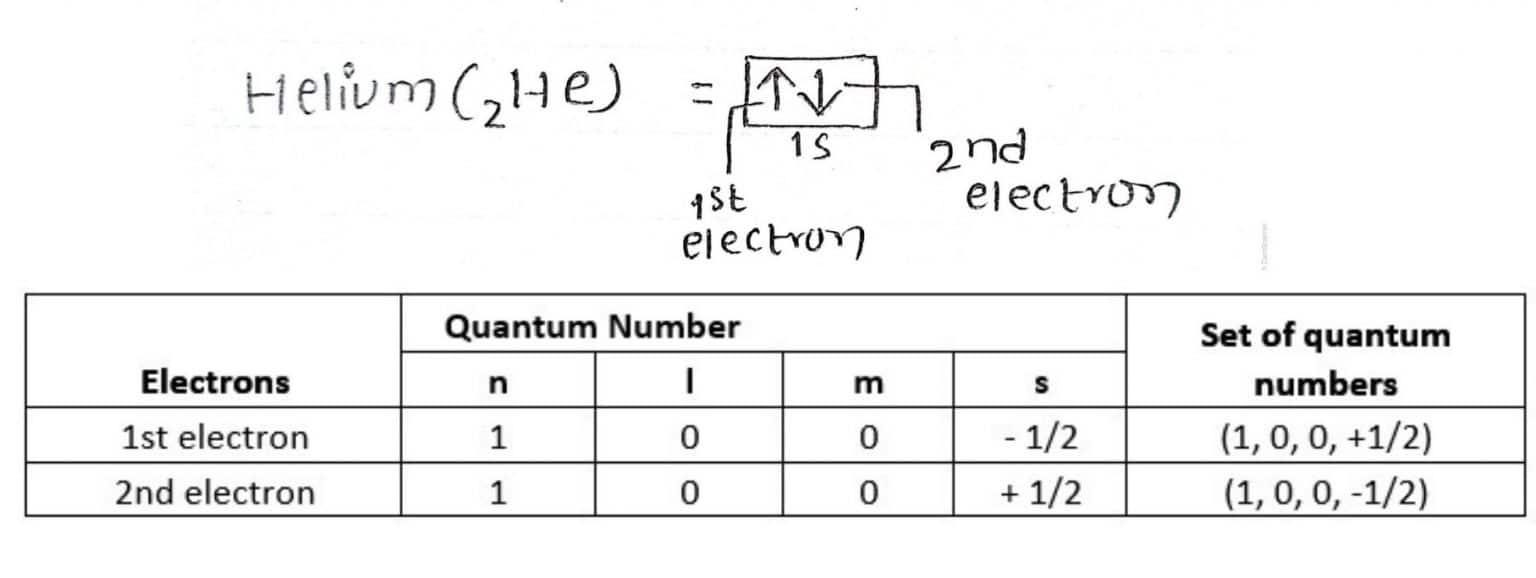
What Is The Difference Between Hund's Rule And Pauli Exclusion Principle . Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. Discover how these inform quantum physics, the. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. One electron goes into each until all of them are half full before pairing up. They explain how electrons arrange themselves. Hund’s rule tells us to place the.
from chemistnotes.com
Hund’s rule tells us to place the. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. One electron goes into each until all of them are half full before pairing up. On the other hand, the. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Discover how these inform quantum physics, the. They explain how electrons arrange themselves.
Pauli Exclusion Principle Definition, Example, and easy Application
What Is The Difference Between Hund's Rule And Pauli Exclusion Principle The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Discover how these inform quantum physics, the. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. On the other hand, the. One electron goes into each until all of them are half full before pairing up. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. They explain how electrons arrange themselves. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Hund’s rule tells us to place the.
From www.youtube.com
What is Pauli's exclusion principle? Rule of Electronic Configuration What Is The Difference Between Hund's Rule And Pauli Exclusion Principle The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. One electron goes into each until all of them are half full before pairing up. On the other hand, the. Pauli’s exclusion principle tells us. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From chemistnotes.com
Pauli Exclusion Principle Definition, Example, and easy Application What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Discover how these inform quantum physics, the. Hund’s rule tells us to place the. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Three rules that help define electron positions within an atom are hund's rule, the. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Aufbau principal Pauli exclusion principle, hund's rule Simplified What Is The Difference Between Hund's Rule And Pauli Exclusion Principle One electron goes into each until all of them are half full before pairing up. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. On the other hand, the. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. They explain how electrons arrange. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From pediaa.com
Difference Between Aufbau Principle and Hund's Rule Theory What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. One electron goes into each until all of them are half full before pairing up. They explain how electrons arrange themselves. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. Discover how. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. They explain how. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From scienceinfo.com
Pauli Exclusion Principle Statement, Examples, Importance What Is The Difference Between Hund's Rule And Pauli Exclusion Principle They explain how electrons arrange themselves. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. The pauli exclusion principle and hund's rules are key concepts in understanding atomic. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
2.3 a Aufbau principle, Hund's rule and Pauli exclusion principle YouTube What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. One electron goes into each until all of them are half full before pairing up. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. Hund’s rule and the pauli exclusion principle are two. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From slideplayer.com
Electron Configuration Orbital Notation LewisElectron Dot Diagram What Is The Difference Between Hund's Rule And Pauli Exclusion Principle One electron goes into each until all of them are half full before pairing up. They explain how electrons arrange themselves. Discover how these inform quantum physics, the. On the other hand, the. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. The pauli exclusion principle. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.vrogue.co
Difference Between Hunds Rule And Pauli Exclusion Pri vrogue.co What Is The Difference Between Hund's Rule And Pauli Exclusion Principle One electron goes into each until all of them are half full before pairing up. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Three rules that help. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle They explain how electrons arrange themselves. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From askfilo.com
The orbital diagram in which both the Pauli"s exclusion principle and Hun.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle They explain how electrons arrange themselves. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Discover how these inform quantum physics, the. Pauli’s exclusion principle. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From pediaa.com
Difference Between Aufbau Principle and Hund's Rule Theory What Is The Difference Between Hund's Rule And Pauli Exclusion Principle On the other hand, the. Hund’s rule tells us to place the. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. They explain how electrons arrange themselves. Hund’s rule and the pauli exclusion principle. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Electronic Configuration Hund's Rule Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Discover how these inform quantum physics, the. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. They explain how electrons arrange themselves. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Three rules that help define electron positions within an atom. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.studypool.com
SOLUTION Pauli exclusion hund s rule and aufbau principle Studypool What Is The Difference Between Hund's Rule And Pauli Exclusion Principle The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. They explain how electrons arrange themselves. On the other hand, the. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Hund’s rule tells us to place the. Three rules that help define electron positions within an atom. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.chemistrylearner.com
Pauli Exclusion Principle Statement, Examples, and Diagrams What Is The Difference Between Hund's Rule And Pauli Exclusion Principle One electron goes into each until all of them are half full before pairing up. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. They explain how electrons arrange themselves. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From vimeo.com
Electron Configuration Rules for Orbital Diagrams (Aufbau, Pauli What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Discover how these inform quantum physics, the. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. In summary, the pauli exclusion principle dictates that no. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Pauli Exclusion Principle and Hund's Rule of Maximum Multiplicity What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. They explain how electrons arrange themselves. Discover how these inform quantum physics, the. In summary, the pauli exclusion principle dictates that. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From chemistnotes.com
Hund's Rule, Definition, Example, and Application Chemistry Notes What Is The Difference Between Hund's Rule And Pauli Exclusion Principle One electron goes into each until all of them are half full before pairing up. Hund’s rule tells us to place the. Discover how these inform quantum physics, the. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. Pauli’s exclusion principle tells us to place the arrows of. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Three rules. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Aufbau principle, Hund's rule & Pauli exclusion principle. YouTube What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. They explain how electrons arrange themselves. Hund’s rule tells us to place the. One electron goes into each until all of them are half full. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From enginelibmisallying.z14.web.core.windows.net
What Is The Basis For Hund's Rule What Is The Difference Between Hund's Rule And Pauli Exclusion Principle In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From chemistnotes.com
Pauli Exclusion Principle Definition, Example, and easy Application What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. One electron goes into each until all of them are half full before pairing up. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Three rules that help define electron positions within an atom are hund's rule,. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.geeksforgeeks.org
Pauli Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. One electron goes into each until all of them are half full before pairing up. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. Discover how these inform quantum physics, the. They explain how electrons arrange themselves.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.tes.com
Pauli Exclusion Principle, Aufbau and Hund's Rule Grade 12 Chemistry What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund’s rule tells us to place the. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. On the other hand, the. They explain how electrons arrange themselves. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. One. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.pinterest.co.uk
Aufbau Principle, Pauli Exclusion Principle and Hund's Rule Aufbau What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. Hund’s rule tells us to place the. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. They explain how electrons arrange themselves. Discover how these inform quantum physics, the. The pauli exclusion principle and hund's rules are key concepts in understanding atomic structure. In summary, the pauli exclusion principle dictates that no two. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From askfilo.com
The orbital diagram in which Pauli exclusion principle, Hund's rule and A.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. On the other. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From sciencenotes.org
Pauli Exclusion Principle What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Discover how these inform quantum physics, the. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. They explain how electrons arrange themselves. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Hund’s rule tells us to place the. Hund's. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.studypool.com
SOLUTION Pauli exclusion hund s rule and aufbau principle Studypool What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up. They explain how electrons arrange themselves. Hund’s rule tells us to place the. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. On the other hand, the. One electron goes. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.youtube.com
Rules For Distribution of Electrons Afbau PrinciplePauli's Exclusion What Is The Difference Between Hund's Rule And Pauli Exclusion Principle They explain how electrons arrange themselves. Discover how these inform quantum physics, the. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. On the other hand, the. Hund’s rule tells. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From sciencenotes.org
Hund's Rule Definition and Examples What Is The Difference Between Hund's Rule And Pauli Exclusion Principle In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund’s rule tells us to place the. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. They explain how electrons arrange themselves. One electron goes. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From pediaa.com
What is the Difference Between Pauli Exclusion Principle and Hund's What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Hund’s rule tells us to place the. Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. On the other hand, the. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The pauli exclusion principle and hund's rules are key. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.slideserve.com
PPT Hund’s Rule PowerPoint Presentation, free download ID562489 What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions. One electron goes into each until all of them are half full before pairing up. Hund’s rule and the pauli exclusion principle are two principles in quantum mechanics that govern the behavior of electrons in atoms. In summary, the pauli exclusion principle. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.
From www.chemistrylearner.com
Hund’s Rule Statement, Definition, and Example. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle Discover how these inform quantum physics, the. On the other hand, the. Hund’s rule tells us to place the. In summary, the pauli exclusion principle dictates that no two electrons in an atom can have the same quantum numbers, while hund's rule. Hund's rule prioritizes the alignment of electron spins, leading to the occupation of separate orbitals before pairing up.. What Is The Difference Between Hund's Rule And Pauli Exclusion Principle.