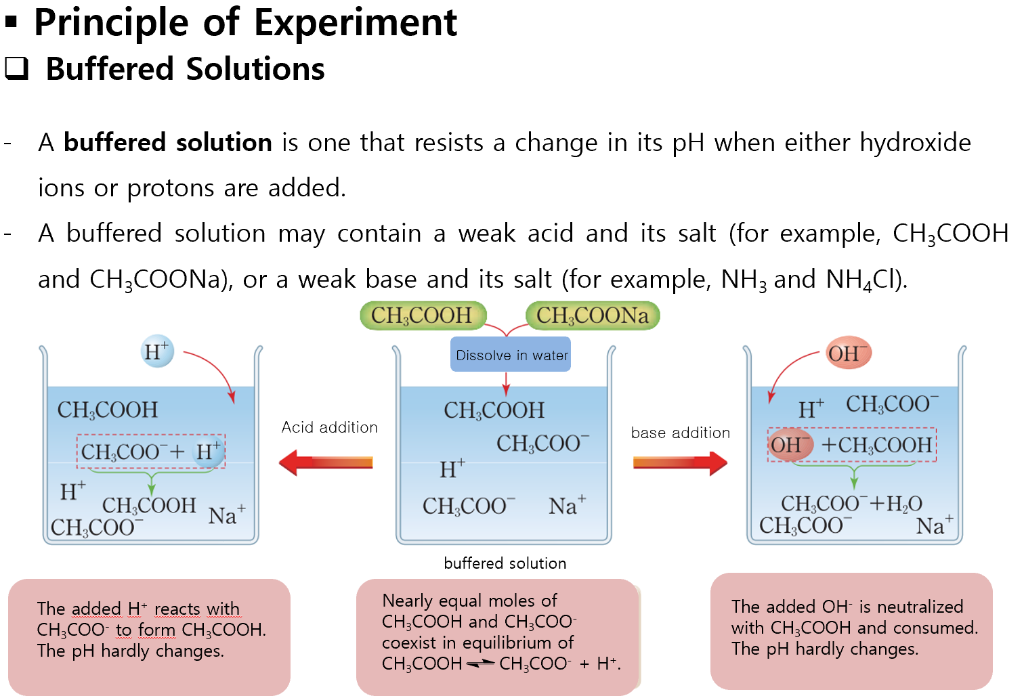
How To Make A Buffer Solution In The Lab . Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. An important point that must be. A buffer solution is a mixture of a weak acid and its. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the.
from allyson-has-mccoy.blogspot.com
An important point that must be. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer solution is a mixture of a weak acid and its.
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. An important point that must be. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt].
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation How To Make A Buffer Solution In The Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Buffer preparation is a common process in chemistry and biochemistry laboratories. A. How To Make A Buffer Solution In The Lab.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high How To Make A Buffer Solution In The Lab Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. An important point that must be. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer is a solution that can resist ph. How To Make A Buffer Solution In The Lab.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation How To Make A Buffer Solution In The Lab A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer solution is a mixture of a weak acid and its. Buffer preparation is a common process in chemistry and biochemistry. How To Make A Buffer Solution In The Lab.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation How To Make A Buffer Solution In The Lab A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Acidic buffer basic buffer are made from weak base and its conjugated acid [. How To Make A Buffer Solution In The Lab.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make A Buffer Solution In The Lab Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer is a solution that resists changes. How To Make A Buffer Solution In The Lab.
From www.brainkart.com
How do buffers work? How To Make A Buffer Solution In The Lab Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus. How To Make A Buffer Solution In The Lab.
From www.youtube.com
Preparation and Analysis of Buffers Intro & Theory YouTube How To Make A Buffer Solution In The Lab Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. An important point that must be. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its. How To Make A Buffer Solution In The Lab.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions How To Make A Buffer Solution In The Lab It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer solution is a mixture of a weak acid and its.. How To Make A Buffer Solution In The Lab.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 How To Make A Buffer Solution In The Lab A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. A buffer solution is a mixture of a weak acid and its. An important point that must be. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A. How To Make A Buffer Solution In The Lab.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk How To Make A Buffer Solution In The Lab A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. An important point that must be. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer preparation is a common process in chemistry and biochemistry laboratories. It is. How To Make A Buffer Solution In The Lab.
From studylib.net
Buffer Solutions How To Make A Buffer Solution In The Lab Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. A buffer solution is a mixture of a weak acid and its. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl. How To Make A Buffer Solution In The Lab.
From www.youtube.com
How to Make and pH Buffers YouTube How To Make A Buffer Solution In The Lab An important point that must be. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Buffer preparation is a common process in chemistry and biochemistry laboratories. Nh/nh 4 cl(pkb) ènh 3 (weak. How To Make A Buffer Solution In The Lab.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps How To Make A Buffer Solution In The Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the.. How To Make A Buffer Solution In The Lab.
From www.youtube.com
how to prepare a buffer with a particular pH YouTube How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that resists changes in ph,. How To Make A Buffer Solution In The Lab.
From www.slideshare.net
2012 topic 18 2 buffer solutions How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. An important point that must be. A buffer is a solution that can resist ph change upon the addition. How To Make A Buffer Solution In The Lab.
From sciencing.com
How to Make a Citric Acid Buffer Solution Sciencing How To Make A Buffer Solution In The Lab An important point that must be. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer solution is a mixture of a weak acid and its. Acidic buffer basic buffer are made from weak base. How To Make A Buffer Solution In The Lab.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy How To Make A Buffer Solution In The Lab Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An important point that must be. A buffer solution is a mixture of a weak acid and its. A. How To Make A Buffer Solution In The Lab.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog How To Make A Buffer Solution In The Lab A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Acidic buffer basic buffer are made from weak base and its conjugated acid [. How To Make A Buffer Solution In The Lab.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Buffer preparation is a common process in chemistry and biochemistry laboratories. An important point that must be. A buffer is a solution that can resist ph change upon the addition. How To Make A Buffer Solution In The Lab.
From chemistryteory.blogspot.com
chemistry How to Prepare Buffer Solutions? How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer preparation is a common process in. How To Make A Buffer Solution In The Lab.
From www.chegg.com
Solved Calculating the pH of a Buffer Solution To prepare a How To Make A Buffer Solution In The Lab Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. An important point that must be. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base.. How To Make A Buffer Solution In The Lab.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded How To Make A Buffer Solution In The Lab A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Acidic buffer basic buffer are made from weak. How To Make A Buffer Solution In The Lab.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation How To Make A Buffer Solution In The Lab It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer solution is a mixture of a weak acid and its. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh. How To Make A Buffer Solution In The Lab.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its How To Make A Buffer Solution In The Lab Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. An important point that must be. A buffer solution is a mixture of a weak acid. How To Make A Buffer Solution In The Lab.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide How To Make A Buffer Solution In The Lab Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists changes in ph, and it generally consists. How To Make A Buffer Solution In The Lab.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation ID2841454 How To Make A Buffer Solution In The Lab An important point that must be. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a mixture of a weak acid and its. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount. How To Make A Buffer Solution In The Lab.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog How To Make A Buffer Solution In The Lab Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer solution is a mixture of a weak acid and its. An important point that must be. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Acidic buffer basic buffer are made from weak base and its conjugated. How To Make A Buffer Solution In The Lab.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. Acidic buffer basic buffer. How To Make A Buffer Solution In The Lab.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid How To Make A Buffer Solution In The Lab It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its salt]. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An important point that must be. A. How To Make A Buffer Solution In The Lab.
From www.pdfprof.com
PDF examples of buffer solutions PDF Télécharger Download How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. An important point that must be. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or base. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its. How To Make A Buffer Solution In The Lab.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 How To Make A Buffer Solution In The Lab A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. A buffer solution is a mixture of a weak acid and its. Buffer preparation is a common process in chemistry and biochemistry laboratories. Acidic buffer basic buffer are made from weak base and its conjugated acid [ its. How To Make A Buffer Solution In The Lab.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution How To Make A Buffer Solution In The Lab Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Nh/nh 4 cl(pkb) ènh 3 (weak base) ènh 4 cl (conjugated. A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and. How To Make A Buffer Solution In The Lab.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs How To Make A Buffer Solution In The Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a mixture of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. An important point that must be. Buffer preparation is a common. How To Make A Buffer Solution In The Lab.
From www.osmosis.org
Making buffer solutions Osmosis How To Make A Buffer Solution In The Lab A buffer is a solution that resists changes in ph, and it generally consists of a weak acid and its conjugate base. A buffer solution is a mixture of a weak acid and its. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition of a small amount of acid or. How To Make A Buffer Solution In The Lab.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How To Make A Buffer Solution In The Lab A buffer solution is a mixture of a weak acid and its. Buffer preparation is a common process in chemistry and biochemistry laboratories. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of the. A buffer solution consists of a conjugate acid/base pair, so it maintains a fairly constant ph value upon addition. How To Make A Buffer Solution In The Lab.