Electrodes Chemical Analysis . At an anode, electrons are produced. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ise has many advantages compared to. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Various electrochemical sensors can be used to measure important analytes in blood. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. They tend to be inexpensive, robust, sensitive and selective.
from www.youtube.com
In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. At an anode, electrons are produced. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. They tend to be inexpensive, robust, sensitive and selective. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Various electrochemical sensors can be used to measure important analytes in blood. Ise has many advantages compared to.
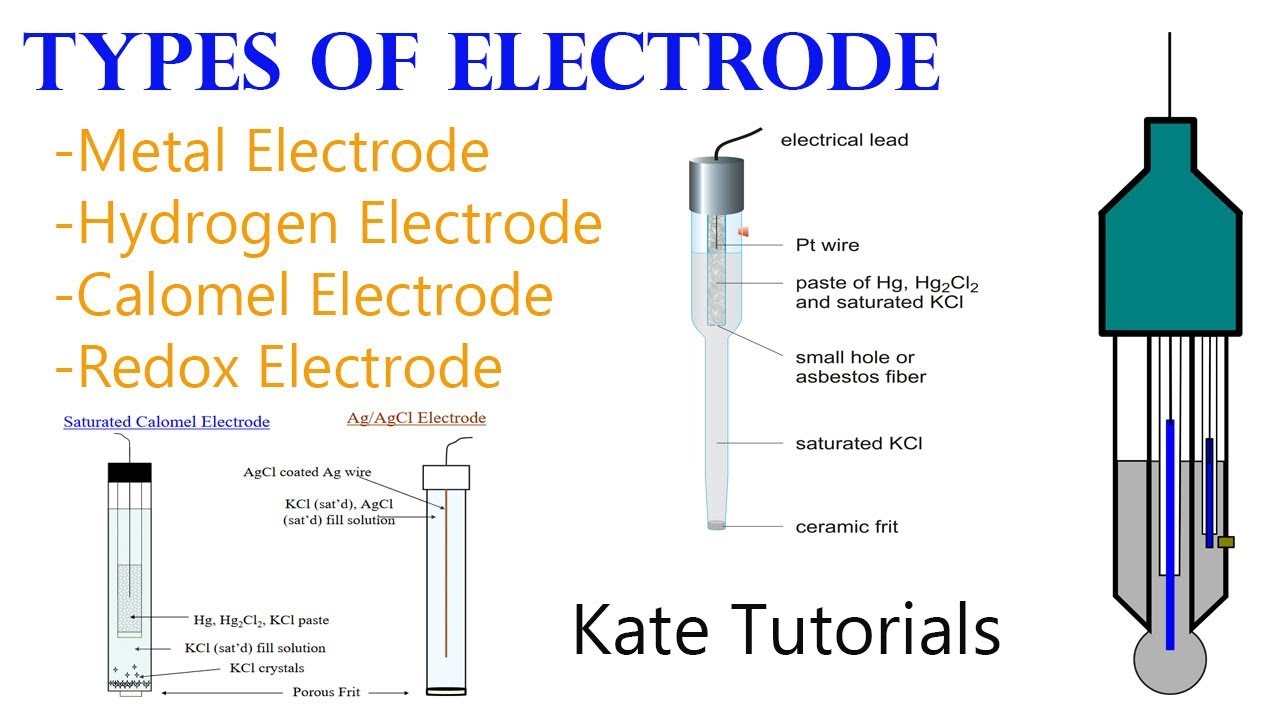
6 Different Types of Electrodes & their Reactions in Electrochemistry
Electrodes Chemical Analysis At an anode, electrons are produced. They tend to be inexpensive, robust, sensitive and selective. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Various electrochemical sensors can be used to measure important analytes in blood. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ise has many advantages compared to. At an anode, electrons are produced. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation.
From www.researchgate.net
Electrochemical detection of hydrogen peroxide using a FTO electrode Electrodes Chemical Analysis At an anode, electrons are produced. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. They tend to be inexpensive, robust, sensitive and selective. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ise. Electrodes Chemical Analysis.
From www.researchgate.net
Chemical composition of the electrodes. Download Scientific Diagram Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. In this chapter we turn our attention to electrochemical. Electrodes Chemical Analysis.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts Electrodes Chemical Analysis Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. They tend to be inexpensive, robust, sensitive and selective. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction. Electrodes Chemical Analysis.
From www.researchgate.net
(A) A threeelectrode cell with the utilization of cyclic voltammetry Electrodes Chemical Analysis Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. They tend to be inexpensive, robust, sensitive and selective. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy. Electrodes Chemical Analysis.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Electrodes Chemical Analysis Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential.. Electrodes Chemical Analysis.
From www.researchgate.net
Electrochemical analysis threeelectrode setup. Schematic of the Electrodes Chemical Analysis Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Various electrochemical sensors can be used. Electrodes Chemical Analysis.
From www.researchgate.net
i Chargedischarge process of a lithiumion cell using graphite and Electrodes Chemical Analysis Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Ise has many advantages compared to. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell.. Electrodes Chemical Analysis.
From www.wcwelding.com
Arc Welding Rods Guide Electrodes Chemical Analysis They tend to be inexpensive, robust, sensitive and selective. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ise has many advantages compared to. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Electrochemical. Electrodes Chemical Analysis.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. At an anode, electrons. Electrodes Chemical Analysis.
From www.dreamstime.com
PH Electrodes Glass Electrode and Working Electrode Stock Image Electrodes Chemical Analysis Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Electrode of an electrochemical. Electrodes Chemical Analysis.
From in.pinterest.com
Electrochemistry Ectrochemical Series Electrochemistry, Chemistry Electrodes Chemical Analysis At an anode, electrons are produced. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Various electrochemical sensors can be used to measure important analytes in blood.. Electrodes Chemical Analysis.
From www.chemistry4.us
IGCSE ChemistryElectrolysis practice Chemistry For Us Electrodes Chemical Analysis They tend to be inexpensive, robust, sensitive and selective. At an anode, electrons are produced. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Various electrochemical sensors can be used to measure important analytes in blood. Ise has many advantages compared to. Electrochemical stripping analysis (esa). Electrodes Chemical Analysis.
From mungfali.com
Glass Electrode Diagram Electrodes Chemical Analysis Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Ise has many advantages compared to. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. At an anode, electrons are produced. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the. Electrodes Chemical Analysis.
From www.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Ise has many advantages compared to. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. They tend to be inexpensive, robust, sensitive and selective. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge. Electrodes Chemical Analysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrodes Chemical Analysis They tend to be inexpensive, robust, sensitive and selective. Various electrochemical sensors can be used to measure important analytes in blood. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. At an anode, electrons are produced. Ise has many advantages compared to. Ion selective electrode (ise) is an analytical technique used to determine. Electrodes Chemical Analysis.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrodes Chemical Analysis Ise has many advantages compared to. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. At an anode, electrons are produced. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Various electrochemical sensors can be used to measure important analytes. Electrodes Chemical Analysis.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Electrodes Chemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. At an anode, electrons are produced. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Ise has many advantages compared to. They tend to be. Electrodes Chemical Analysis.
From www.chegg.com
Solved Chapter 17 Problem 4P Solution Exploring Chemical Analysis Electrodes Chemical Analysis They tend to be inexpensive, robust, sensitive and selective. At an anode, electrons are produced. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Various. Electrodes Chemical Analysis.
From www.researchgate.net
Cyclic voltammograms of the gold electrode in the 1 M H2SO4 solution Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis). Electrodes Chemical Analysis.
From kindle-tech.com
Electrochemical Electrodes In Chemical Analysis Kintek Solution Electrodes Chemical Analysis Ise has many advantages compared to. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Various electrochemical sensors can be used to measure important analytes in blood. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Electrode of an electrochemical. Electrodes Chemical Analysis.
From www.researchgate.net
Covered electrode weld metal chemical composition Download Table Electrodes Chemical Analysis Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. They tend to be inexpensive, robust, sensitive and selective. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy. Electrodes Chemical Analysis.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Electrodes Chemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Various electrochemical sensors can be used to measure important analytes in blood. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Cyclic voltammetry (cv) and. Electrodes Chemical Analysis.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Ise has many advantages compared to. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. At an anode, electrons. Electrodes Chemical Analysis.
From www.ravindraheraeus.com
Platinum Electrodes Ravindra Heraeus Electrodes Chemical Analysis Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. At an anode, electrons are produced. They tend to be inexpensive, robust, sensitive and selective. Ise has many advantages compared. Electrodes Chemical Analysis.
From blog.rheosense.com
Characterize Your Battery Slurry with the Right Tools Electrodes Chemical Analysis At an anode, electrons are produced. Ise has many advantages compared to. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. They tend to be inexpensive, robust, sensitive and selective. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. In this. Electrodes Chemical Analysis.
From fjelloghjem.blogspot.com
18 Fresh Piston Engine Diagram Electrodes Chemical Analysis At an anode, electrons are produced. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Various electrochemical sensors can be used to measure important analytes. Electrodes Chemical Analysis.
From www.researchgate.net
Electrode chemical composition Download Scientific Diagram Electrodes Chemical Analysis Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Various electrochemical sensors can be used to measure important analytes in blood. Ise has many advantages. Electrodes Chemical Analysis.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis). Electrodes Chemical Analysis.
From chemistry-europe.onlinelibrary.wiley.com
Understanding Electrolyte Filling of Lithium‐Ion Battery Electrodes on Electrodes Chemical Analysis At an anode, electrons are produced. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination. Electrodes Chemical Analysis.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Electrodes Chemical Analysis Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Various electrochemical sensors can be used to measure important analytes in blood. Ion selective electrode (ise) is an analytical technique used to. Electrodes Chemical Analysis.
From www.researchgate.net
Impermeable covered electrode weld metal chemical composition Electrodes Chemical Analysis Various electrochemical sensors can be used to measure important analytes in blood. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Ise has many advantages compared to. Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Cyclic voltammetry (cv) and. Electrodes Chemical Analysis.
From pineresearch.com
ThreeElectrode Setups Pine Research Instrumentation Store Electrodes Chemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. They tend to be inexpensive, robust, sensitive and selective. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Ise has many advantages compared to. Various electrochemical sensors. Electrodes Chemical Analysis.
From pubs.rsc.org
An environmentfriendly crosslinked binder endowing LiFePO 4 electrode Electrodes Chemical Analysis At an anode, electrons are produced. They tend to be inexpensive, robust, sensitive and selective. Ise has many advantages compared to. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for.. Electrodes Chemical Analysis.
From publishing.aip.org
Improving HighEnergy LithiumIon Batteries with Carbon Filler AIP Electrodes Chemical Analysis Electrochemical stripping analysis (esa) is a trace electroanalytical technique for the determination of metal cations, inorganic ions,. Cyclic voltammetry (cv) and electrochemical impedance spectroscopy (eis) are some of the most common techniques for. Various electrochemical sensors can be used to measure important analytes in blood. Electrode of an electrochemical cell through which net electric current flows and at which the. Electrodes Chemical Analysis.
From www.philipharris.co.uk
B8R05772 Mounted Carbon Electrodes Philip Harris Electrodes Chemical Analysis Ise has many advantages compared to. At an anode, electrons are produced. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an electrochemical cell. Electrode of an electrochemical cell through which net electric current flows and at which the predominating electrochemical reaction is an oxidation. Electrochemical stripping analysis (esa) is a. Electrodes Chemical Analysis.