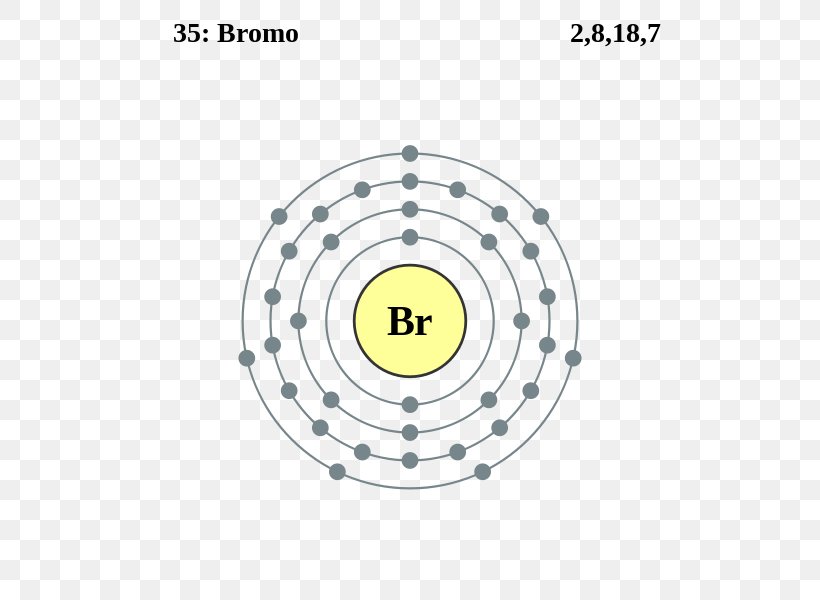
Electron Configuration For Bromine Ion . The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you go. Determine the electron configuration of ions. This can be shortened to [ar]4s23d104p5. The properties of chlorine are intermediate between those of iodine and chlorine. Melting point the temperature at which the. The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Electron configuration chart of all elements is mentioned in the table below. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The full version of this is: Use a chart such as the one below. Justify the observed charge of ions to their electronic configuration.
from favpng.com
Melting point the temperature at which the. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. This can be shortened to [ar]4s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you go. Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Use a chart such as the one below.
Electron Configuration Bromine Chemical Element Electron Shell Bohr
Electron Configuration For Bromine Ion The shorthand electron configuration (or noble gas configuration) as well as. The properties of chlorine are intermediate between those of iodine and chlorine. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below. Use a chart such as the one below. All you need to do is work your way across the periodic table filling the orbitals as you go. Justify the observed charge of ions to their electronic configuration. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Melting point the temperature at which the. Determine the electron configuration of ions. The full version of this is: This can be shortened to [ar]4s23d104p5. The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Electron Configuration For Bromine Ion Justify the observed charge of ions to their electronic configuration. This can be shortened to [ar]4s23d104p5. Melting point the temperature at which the. The full version of this is: Determine the electron configuration of ions. The properties of chlorine are intermediate between those of iodine and chlorine. The shorthand electron configuration (or noble gas configuration) as well as. All you. Electron Configuration For Bromine Ion.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Determine the electron configuration of ions. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble. Electron Configuration For Bromine Ion.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. Melting point the temperature at which the. The shorthand electron configuration (or noble gas configuration) as well as. All you need to do is work your way across the periodic table filling the orbitals as you go. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2. Electron Configuration For Bromine Ion.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Electron Configuration For Bromine Ion The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Determine the electron configuration of ions. The properties of chlorine are intermediate between those of iodine and chlorine. All you need to do is work your way across. Electron Configuration For Bromine Ion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Electron Configuration For Bromine Ion Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The properties of chlorine are intermediate between those of iodine and chlorine. The shorthand electron configuration (or noble gas configuration) as well as. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. Determine the electron configuration of ions. All you need to. Electron Configuration For Bromine Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Configuration For Bromine Ion Determine the electron configuration of ions. All you need to do is work your way across the periodic table filling the orbitals as you go. This can be shortened to [ar]4s23d104p5. Justify the observed charge of ions to their electronic configuration. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The full version of this is: The shorthand electron configuration (or noble. Electron Configuration For Bromine Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Configuration For Bromine Ion Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Use a chart such as the one below. The full version of this is: Determine the electron configuration of ions. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2. Electron Configuration For Bromine Ion.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Electron Configuration For Bromine Ion Determine the electron configuration of ions. Melting point the temperature at which the. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below. All you need to do is work your way across the periodic table filling the orbitals as you go. The properties of chlorine are intermediate between those of. Electron Configuration For Bromine Ion.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Electron Configuration For Bromine Ion The shorthand electron configuration (or noble gas configuration) as well as. This can be shortened to [ar]4s23d104p5. The properties of chlorine are intermediate between those of iodine and chlorine. Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The full version of this is: Determine the electron configuration of ions. Melting point the temperature. Electron Configuration For Bromine Ion.
From schematicpiscardixf.z4.web.core.windows.net
Orbital Diagram For Bromine Electron Configuration For Bromine Ion Determine the electron configuration of ions. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Melting point the temperature at which the. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration. Electron Configuration For Bromine Ion.
From exolwghqz.blob.core.windows.net
Bromide Formula For Sodium at Emilio Canon blog Electron Configuration For Bromine Ion Melting point the temperature at which the. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The full version of this is: The properties of chlorine are intermediate between those of iodine and chlorine. Justify the observed charge of ions to their electronic configuration. The. Electron Configuration For Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration For Bromine Ion Use a chart such as the one below. The shorthand electron configuration (or noble gas configuration) as well as. Determine the electron configuration of ions. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. All you need to do is work your way across the periodic table filling the. Electron Configuration For Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration For Bromine Ion Justify the observed charge of ions to their electronic configuration. The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Melting point the temperature at which the. All you need to do is work. Electron Configuration For Bromine Ion.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Electron Configuration For Bromine Ion The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. The properties of chlorine are intermediate between those of iodine and chlorine. Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. The shorthand electron configuration (or noble gas. Electron Configuration For Bromine Ion.
From brainly.com
Which is the electron configuration for bromine? Electron Configuration For Bromine Ion The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. This can be shortened to [ar]4s23d104p5. All you need to do is work your way across the periodic table filling the orbitals. Electron Configuration For Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration For Bromine Ion Electron configuration chart of all elements is mentioned in the table below. Determine the electron configuration of ions. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. The properties of chlorine are intermediate between those of iodine and chlorine. The full version of this. Electron Configuration For Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. Justify the observed charge of ions to their electronic configuration. Electron configuration chart of all elements is mentioned in the table below. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The full version of this is: Use a chart such as the one. Electron Configuration For Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration For Bromine Ion Melting point the temperature at which the. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Determine the electron configuration of ions. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or. Electron Configuration For Bromine Ion.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Electron Configuration For Bromine Ion Electron configuration chart of all elements is mentioned in the table below. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Melting point the temperature at which the. Determine the electron configuration of ions. The full version of this is: The bromine electron configuration, denoted as 4s2 3d10. Electron Configuration For Bromine Ion.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Electron Configuration For Bromine Ion Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Determine the electron configuration of ions. All you need to do is work your way. Electron Configuration For Bromine Ion.
From wiringdbestevezrealmsx.z13.web.core.windows.net
Dot And Cross Diagram For Sodium Chloride Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. This can be shortened to [ar]4s23d104p5. The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Justify the observed charge of ions. Electron Configuration For Bromine Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Configuration For Bromine Ion Electron configuration chart of all elements is mentioned in the table below. Justify the observed charge of ions to their electronic configuration. The shorthand electron configuration (or noble gas configuration) as well as. Melting point the temperature at which the. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The properties of chlorine are intermediate between. Electron Configuration For Bromine Ion.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. Determine the electron configuration of ions. This can be shortened to [ar]4s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you go. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Electron Configuration For Bromine Ion.
From brodskymuchey.blogspot.com
Same Electron Configuration as the Bromide Ion Br Brodsky Muchey Electron Configuration For Bromine Ion The full version of this is: Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Justify the observed charge of ions to their electronic configuration. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The properties of chlorine are intermediate between those of iodine and chlorine. This can be shortened to [ar]4s23d104p5. All you need to do is. Electron Configuration For Bromine Ion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Electron Configuration For Bromine Ion This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Melting point the temperature at which the. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The shorthand electron configuration (or noble gas configuration) as. Electron Configuration For Bromine Ion.
From brodskymuchey.blogspot.com
Same Electron Configuration as the Bromide Ion Br Brodsky Muchey Electron Configuration For Bromine Ion Use a chart such as the one below. Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. Melting point the temperature at which the. Electron configuration chart of all elements is mentioned in the table below. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The properties of chlorine. Electron Configuration For Bromine Ion.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Electron Configuration For Bromine Ion Melting point the temperature at which the. Use a chart such as the one below. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The properties of chlorine are intermediate between those of iodine and chlorine. The bromine electron configuration, denoted as 4s2 3d10 4p5. Electron Configuration For Bromine Ion.
From fyodahncv.blob.core.windows.net
Magnesium Bromide Molecular Formula at Francis Rogers blog Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. All you need to do is work your way across the periodic table filling the orbitals as you go. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The full version of this is: Justify the observed. Electron Configuration For Bromine Ion.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electron Configuration For Bromine Ion Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. Use a chart such as the one below. The shorthand electron configuration (or noble gas configuration) as well as. This can be shortened to [ar]4s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The full version of this is:. Electron Configuration For Bromine Ion.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Electron Configuration For Bromine Ion The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Use a chart such as the one below. The properties of chlorine are intermediate between those of iodine and chlorine. The shorthand electron configuration (or noble gas configuration) as well as. Justify the observed charge. Electron Configuration For Bromine Ion.
From br.pinterest.com
Electronic Configuration of Bromine,Br Electron configuration Electron Configuration For Bromine Ion Electron configuration chart of all elements is mentioned in the table below. Justify the observed charge of ions to their electronic configuration. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Determine the electron configuration of ions.. Electron Configuration For Bromine Ion.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration For Bromine Ion Melting point the temperature at which the. The shorthand electron configuration (or noble gas configuration) as well as. This can be shortened to [ar]4s23d104p5. The properties of chlorine are intermediate between those of iodine and chlorine. Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. All you need to do is work your. Electron Configuration For Bromine Ion.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Electron Configuration For Bromine Ion The properties of chlorine are intermediate between those of iodine and chlorine. The shorthand electron configuration (or noble gas configuration) as well as. Determine the electron configuration of ions. Electron configuration chart of all elements is mentioned in the table below. Melting point the temperature at which the. Use a chart such as the one below. This can be shortened. Electron Configuration For Bromine Ion.
From schematicfixgingles.z21.web.core.windows.net
Orbital Energy Diagram For The Cobalt Iii Ion Electron Configuration For Bromine Ion Use a chart such as the one below. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. The shorthand electron configuration (or noble gas configuration) as well as. All you need to do is work your way across. Electron Configuration For Bromine Ion.
From manualfixthanedom77.z22.web.core.windows.net
Sodium Oxide Lewis Diagram Electron Configuration For Bromine Ion Melting point the temperature at which the. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within. Determine the electron configuration of ions. All you need to do is work your way across the periodic table filling the. Electron Configuration For Bromine Ion.