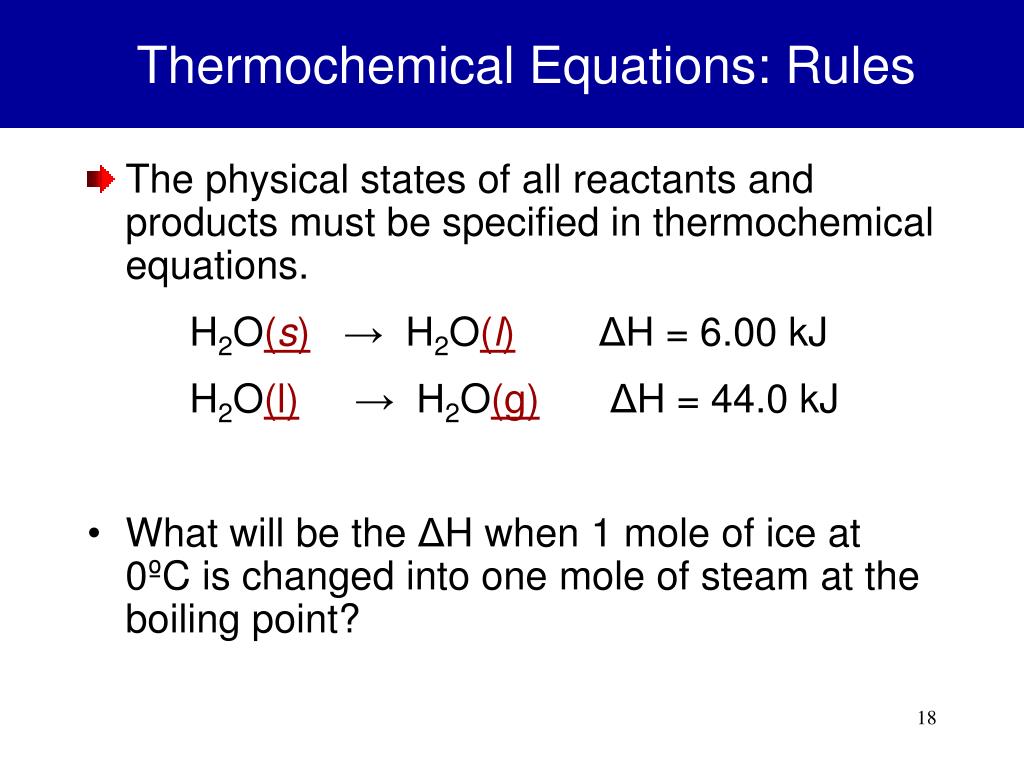
Thermochem Formulas . Thermochemistry deals with heat (energy) changes in chemical reactions. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. In chemical reactions heat is released or absorbed. We can express the first law in many ways. Q can be positive or negative, energy. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. It provides a list of formulas. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Temperature is in celsius not kelvins for this topic! This chemistry video lecture tutorial focuses on thermochemistry.
from www.slideserve.com
Temperature is in celsius not kelvins for this topic! A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Thermochemistry deals with heat (energy) changes in chemical reactions. It provides a list of formulas. In chemical reactions heat is released or absorbed. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. This chemistry video lecture tutorial focuses on thermochemistry. Q can be positive or negative, energy. We can express the first law in many ways.
PPT Thermochemistry PowerPoint Presentation, free download ID5405762
Thermochem Formulas It provides a list of formulas. This chemistry video lecture tutorial focuses on thermochemistry. In chemical reactions heat is released or absorbed. We can express the first law in many ways. Thermochemistry deals with heat (energy) changes in chemical reactions. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. Q can be positive or negative, energy. Temperature is in celsius not kelvins for this topic! It provides a list of formulas.
From www.studypool.com
SOLUTION Thermodynamics formula sheet 1 Studypool Thermochem Formulas In chemical reactions heat is released or absorbed. We can express the first law in many ways. This chemistry video lecture tutorial focuses on thermochemistry. Temperature is in celsius not kelvins for this topic! It provides a list of formulas. Thermochemistry deals with heat (energy) changes in chemical reactions. Q can be positive or negative, energy. Here the δ hm. Thermochem Formulas.
From www.slideserve.com
PPT OB Students will develop a mastery of all thermochem math Thermochem Formulas Temperature is in celsius not kelvins for this topic! This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. In chemical reactions heat is released or absorbed. This chemistry video lecture tutorial focuses on thermochemistry. We can express the first law in many ways. Q can be positive or negative, energy. One. Thermochem Formulas.
From www.formsbank.com
Thermodynamics Formulas printable pdf download Thermochem Formulas One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Temperature is in celsius not kelvins for this topic! A reaction may absorb or release energy and phase change may include the same namely. Thermochem Formulas.
From www.studocu.com
Chem 30 Formulas 123 Chemistry 30 Formulas and Information Thermochem Formulas It provides a list of formulas. We can express the first law in many ways. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. This chapter introduces you to thermochemistry,. Thermochem Formulas.
From ar.inspiredpencil.com
Thermodynamics Formula Sheet Thermochem Formulas It provides a list of formulas. We can express the first law in many ways. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Q can be positive or negative, energy. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Temperature is. Thermochem Formulas.
From www.differencebetween.com
What is the Difference Between Thermochemical Equation and Chemical Thermochem Formulas Q can be positive or negative, energy. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. This chemistry video lecture tutorial focuses on thermochemistry. We can express the. Thermochem Formulas.
From www.slideserve.com
PPT Unit 3 Thermochemistry PowerPoint Presentation, free download Thermochem Formulas It provides a list of formulas. We can express the first law in many ways. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. This chemistry video lecture tutorial focuses on thermochemistry. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy. Thermochem Formulas.
From www.scribd.com
Chem100 Thermochem Calorie Heat Thermochem Formulas A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. In chemical reactions heat is released or absorbed. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Q can be positive or negative, energy. This chemistry video lecture tutorial focuses on thermochemistry.. Thermochem Formulas.
From harry-ainlay.ca
Thermochemical Equations / Slide10.JPG Thermochem Formulas We can express the first law in many ways. This chemistry video lecture tutorial focuses on thermochemistry. Thermochemistry deals with heat (energy) changes in chemical reactions. Q can be positive or negative, energy. In chemical reactions heat is released or absorbed. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any. Thermochem Formulas.
From studylib.net
Thermochem Slideshow Thermochem Formulas A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. It provides a list of formulas. Thermochemistry deals with heat (energy) changes in chemical reactions. In chemical reactions heat is released or absorbed. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions.. Thermochem Formulas.
From www.slideserve.com
PPT Unit 3 Thermochemistry PowerPoint Presentation, free download Thermochem Formulas Q can be positive or negative, energy. In chemical reactions heat is released or absorbed. This chemistry video lecture tutorial focuses on thermochemistry. We can express the first law in many ways. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. This chapter introduces you to thermochemistry, a. Thermochem Formulas.
From thermochem.weebly.com
Examples Thermochemistry Thermochem Formulas This chemistry video lecture tutorial focuses on thermochemistry. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Thermochemistry deals with heat (energy) changes in chemical reactions. Q can be positive or negative, energy. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process.. Thermochem Formulas.
From www.vrogue.co
Basic Thermodynamic Formulas Exam Equation Sheet Chea vrogue.co Thermochem Formulas Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. In chemical reactions heat is released or absorbed. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. One of the more useful expressions is that the change in internal energy, ∆e, of. Thermochem Formulas.
From www.youtube.com
How to Calculate Specific Heat (Thermochemistry) YouTube Thermochem Formulas Temperature is in celsius not kelvins for this topic! This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. In chemical reactions heat is released or absorbed. Thermochemistry deals with heat (energy) changes in chemical reactions. We can express the first law in many ways. One of the more useful expressions is. Thermochem Formulas.
From studylib.net
thermochemical equation Thermochem Formulas Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. Thermochemistry deals with heat (energy) changes in chemical reactions. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. One of the more useful expressions is that the change. Thermochem Formulas.
From www.docsity.com
Thermochemistry formula sheet Cheat Sheet Chemistry Docsity Thermochem Formulas A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. Temperature is in celsius not kelvins for this topic! One of the more useful expressions is. Thermochem Formulas.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Thermochem Formulas We can express the first law in many ways. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Thermochemistry deals with heat (energy) changes in chemical. Thermochem Formulas.
From www.scribd.com
Thermochem Thermodynamics PDF Heat Heat Capacity Thermochem Formulas It provides a list of formulas. This chemistry video lecture tutorial focuses on thermochemistry. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Temperature is in celsius not kelvins for this topic! We. Thermochem Formulas.
From www.youtube.com
Chapter 06 Thermochemistry Part II YouTube Thermochem Formulas One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. We can express the first law in many ways. It provides a list of formulas. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. In chemical. Thermochem Formulas.
From www.youtube.com
Thermochem Part 3 Thermochemical Equations YouTube Thermochem Formulas It provides a list of formulas. This chemistry video lecture tutorial focuses on thermochemistry. We can express the first law in many ways. Q can be positive or negative, energy. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. In chemical reactions heat is released or absorbed. Thermochemistry. Thermochem Formulas.
From www.youtube.com
Thermochemical Equations YouTube Thermochem Formulas In chemical reactions heat is released or absorbed. It provides a list of formulas. Thermochemistry deals with heat (energy) changes in chemical reactions. Q can be positive or negative, energy. Temperature is in celsius not kelvins for this topic! Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs. Thermochem Formulas.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Thermochem Formulas In chemical reactions heat is released or absorbed. This chemistry video lecture tutorial focuses on thermochemistry. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Temperature is in celsius not kelvins for this topic! Thermochemistry deals with heat (energy) changes in chemical reactions. Q can be positive or. Thermochem Formulas.
From www.youtube.com
Thermodynamics Gibbs free energy, equilibrium and phase change YouTube Thermochem Formulas Temperature is in celsius not kelvins for this topic! Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. One of the more useful expressions is that the change in internal energy, ∆e,. Thermochem Formulas.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2488208 Thermochem Formulas One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. We can express the first law in many ways. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. A reaction may absorb or release energy and. Thermochem Formulas.
From kunduz.com
[ANSWERED] GBE Thermochem The thermochemical equation for the reaction Thermochem Formulas Temperature is in celsius not kelvins for this topic! A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. It provides a list of formulas. Q can be positive or negative, energy. We. Thermochem Formulas.
From www.youtube.com
Thermochemical Equations YouTube Thermochem Formulas In chemical reactions heat is released or absorbed. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. A reaction may absorb or release energy and phase. Thermochem Formulas.
From www.youtube.com
Thermochemical Equations and Using the energy term (heat of reaction Thermochem Formulas Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Temperature is in celsius not kelvins for this topic! This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. In chemical reactions heat is released or absorbed. It provides a list of formulas. Thermochemistry deals with. Thermochem Formulas.
From studylib.net
Thermodynamics Formulas Thermochem Formulas Q can be positive or negative, energy. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. Thermochemistry deals with heat (energy) changes in chemical reactions. Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. Temperature is in celsius not. Thermochem Formulas.
From www.youtube.com
Thermochemical Equations YouTube Thermochem Formulas Q can be positive or negative, energy. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as written,. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. Thermochemistry involves a study of heat and energy related to. Thermochem Formulas.
From www.youtube.com
Thermochemistry Equations and Formulas With Practice Problems YouTube Thermochem Formulas Temperature is in celsius not kelvins for this topic! Q can be positive or negative, energy. Thermochemistry deals with heat (energy) changes in chemical reactions. One of the more useful expressions is that the change in internal energy, ∆e, of a system in any process. In chemical reactions heat is released or absorbed. Here the δ hm (delta h subscript. Thermochem Formulas.
From www.youtube.com
Thermochemical Equations YouTube Thermochem Formulas In chemical reactions heat is released or absorbed. Temperature is in celsius not kelvins for this topic! A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Here the δ hm (delta h subscript m) tells us whether heat energy is released or absorbed when the reaction occurs as. Thermochem Formulas.
From www.bartleby.com
Answered Based on the thermochemical equation… bartleby Thermochem Formulas Temperature is in celsius not kelvins for this topic! A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Q can be positive or negative, energy. Thermochemistry deals with heat (energy) changes in chemical reactions. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy. Thermochem Formulas.
From www.slideserve.com
PPT Thermochemical Equations PowerPoint Presentation, free download Thermochem Formulas Temperature is in celsius not kelvins for this topic! Thermochemistry involves a study of heat and energy related to various physical transformations and chemical reactions. We can express the first law in many ways. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. Q can be positive or. Thermochem Formulas.
From www.youtube.com
ThermoChem Day 3 (Heating Curve Calculations) YouTube Thermochem Formulas We can express the first law in many ways. Q can be positive or negative, energy. Thermochemistry deals with heat (energy) changes in chemical reactions. This chemistry video lecture tutorial focuses on thermochemistry. A reaction may absorb or release energy and phase change may include the same namely boiling and melting, learn more @byjus. One of the more useful expressions. Thermochem Formulas.
From www.slideserve.com
PPT Chapter 11 Thermochemistry PowerPoint Presentation, free download Thermochem Formulas Thermochemistry deals with heat (energy) changes in chemical reactions. It provides a list of formulas. In chemical reactions heat is released or absorbed. This chemistry video lecture tutorial focuses on thermochemistry. This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during. We can express the first law in many ways. One of. Thermochem Formulas.