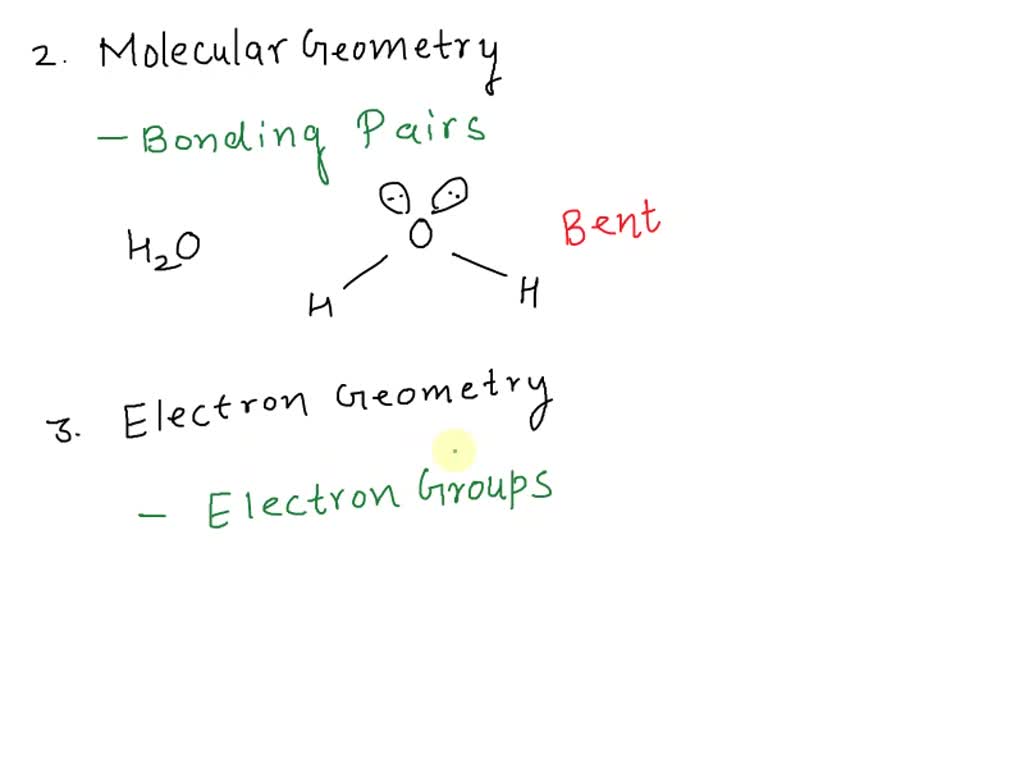
Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices . There must be an odd number of polar bonds so that their polarities not cancel. B) a molecule with symmetrically arranged polar bonds can be. The polarity of a molecule. Lone (unshared) pairs of electrons on the central. The polarity of the molecule. Which of the following statements is correct? If a molecule contains more than one polar bond, it is a polar molecule d. Undqual sharing of bonding electron pairs is what. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Which of the following statements could be true regarding polar molecules? A polar molecule may have one or more lone pairs. Leads to polar bonds within a molecule. If a molecule contains one polar bond, it is a polar molecule c. A)there must be an odd number of polar bonds so that their polarities not cancel. If a molecule contains no dipole moments across any bonds it will be.
from www.numerade.com
Undqual sharing of bonding electron pairs is what. Which of the following statements could be true regarding polar molecules? Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. If a molecule contains no dipole moments across any bonds it will be. Lone (unshared) pairs of electrons on the central. A polar molecule may have one or more lone pairs. If the diatomic molecule’s bond is polar, it is polar. If a molecule contains more than one polar bond, it is a polar molecule d. The polarity of the molecule. Which of the following statements is correct?
SOLVED Which statement is always true according to VSEPR theory? Group
Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Which of the following statements could be true regarding polar molecules? Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. Which of the following statements is correct? Undqual sharing of bonding electron pairs is what. A polar molecule may have one or more lone pairs. B) a molecule with symmetrically arranged polar bonds can be. If a molecule contains more than one polar bond, it is a polar molecule d. Lone (unshared) pairs of electrons on the central. The polarity of a molecule. There must be an odd number of polar bonds so that their polarities not cancel. Which of the following statements could be true regarding polar molecules? A)there must be an odd number of polar bonds so that their polarities not cancel. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. The polarity of the molecule. If a molecule contains one polar bond, it is a polar molecule c. If a molecule contains no dipole moments across any bonds it will be.
From www.toppr.com
Which of the following statements is/are correct? 1) CIF3 is a planar Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Leads to polar bonds within a molecule. A)there must be an odd number of polar bonds so that their polarities not cancel. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. Undqual sharing of bonding electron pairs is what. There. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Which statement is true regarding a polar molecule?A Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains more than one polar bond, it is a polar molecule d. Undqual sharing of bonding electron pairs is what. The polarity of the molecule. A polar molecule may have one or more lone pairs. If a molecule contains no dipole moments across any bonds it will be. For a molecule that has polar bonds, the molecular. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVEDAnswer true or false. (a) To predict whether a covalent molecule Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Leads to polar bonds within a molecule. A polar molecule may have one or more lone pairs. The polarity of a molecule. A)there must be an odd number of polar bonds so that their polarities not cancel. The polarity of. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Question 2 (1 point) Which of the following molecules Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains one polar bond, it is a polar molecule c. The polarity of a molecule. B) a molecule with symmetrically arranged polar bonds can be. Which of the following statements is correct? The polarity of the molecule. If a molecule contains more than one polar bond, it is a polar molecule d. There must be an odd. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVEDUsing your knowledge of dipoles, CF4 … Group of answer choices Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices The polarity of the molecule. If a molecule contains more than one polar bond, it is a polar molecule d. Which of the following statements is correct? If the diatomic molecule’s bond is polar, it is polar. If a molecule contains one polar bond, it is a polar molecule c. A polar molecule may have one or more lone pairs.. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Statements Compounds Example for a polar molecule ICsH12 Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices There must be an odd number of polar bonds so that their polarities not cancel. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. B) a molecule with symmetrically arranged polar bonds can be. If a molecule contains more than. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Question 1 2 pts Which of the following statements about polar Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If the diatomic molecule’s bond is polar, it is polar. Undqual sharing of bonding electron pairs is what. B) a molecule with symmetrically arranged polar bonds can be. A)there must be an odd number of polar bonds so that their polarities not cancel. The polarity of the molecule. If a molecule contains more than one polar bond, it is a. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Which of the following statements is false about SOz and COz ? Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Leads to polar bonds within a molecule. Lone (unshared) pairs of electrons on the central. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Which of the following statements is about polarity? Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices There must be an odd number of polar bonds so that their polarities not cancel. Which of the following statements could be true regarding polar molecules? If a molecule contains one polar bond, it is a polar molecule c. The polarity of the molecule. B) a molecule with symmetrically arranged polar bonds can be. The polarity of a molecule. Lone. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Which statement is always true according to VSEPR theory? Group Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains more than one polar bond, it is a polar molecule d. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. Leads to polar bonds within a molecule. Lone (unshared) pairs of electrons on the central. If. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Formaldehyde on the left is a polar molecule . Is the following Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices B) a molecule with symmetrically arranged polar bonds can be. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Leads to polar bonds within a molecule. Which of the following statements could be true regarding polar molecules? Lone (unshared) pairs of electrons on the central. If a molecule contains. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED 1. Which of the following is a nonpolar molecule? Group of Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. If a molecule contains more than one polar bond, it is a polar molecule d. There must be an odd number of polar bonds so that their polarities not cancel. A. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Which of the following statements are false about chemical Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains more than one polar bond, it is a polar molecule d. Lone (unshared) pairs of electrons on the central. If a molecule contains no dipole moments across any bonds it will be. A)there must be an odd number of polar bonds so that their polarities not cancel. Undqual sharing of bonding electron pairs is what. A. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED 1. Use the below Lewis structures for silicon tetrachloride Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices A polar molecule may have one or more lone pairs. If a molecule contains no dipole moments across any bonds it will be. The polarity of the molecule. Leads to polar bonds within a molecule. Undqual sharing of bonding electron pairs is what. For a molecule that has polar bonds, the molecular geometry must be known in order to predict. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Formaldehyde on the left is a polar molecule . Is the following Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains one polar bond, it is a polar molecule c. A polar molecule may have one or more lone pairs. Leads to polar bonds within a molecule. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. If a molecule contains no dipole moments across any bonds. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Which of the following is true about polarity? Select Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains no dipole moments across any bonds it will be. The polarity of the molecule. There must be an odd number of polar bonds so that their polarities not cancel. A polar molecule may have one or more lone pairs. If a molecule contains more than one polar bond, it is a polar molecule d. Polarity of. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED The hydrogen halides (HF, HCl, HBr, and HI) are all polar Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Which of the following statements could be true regarding polar molecules? Which of the following statements is correct? A)there must be an odd number of polar bonds so that their polarities not cancel. The polarity of a molecule. Leads to polar bonds within a molecule. For a molecule that has polar bonds, the molecular geometry must be known in order. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Which of the following statements about molecular polarity are Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices There must be an odd number of polar bonds so that their polarities not cancel. If the diatomic molecule’s bond is polar, it is polar. Which of the following statements is correct? Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.toppr.com
Given the correct order of initials T or F the following statements Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices A polar molecule may have one or more lone pairs. The polarity of the molecule. If a molecule contains more than one polar bond, it is a polar molecule d. Which of the following statements is correct? There must be an odd number of polar bonds so that their polarities not cancel. Which of the following statements could be true. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Choose all that apply. From the statements below, choose all Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices A polar molecule may have one or more lone pairs. B) a molecule with symmetrically arranged polar bonds can be. Lone (unshared) pairs of electrons on the central. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. Leads to polar. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved The following molecules contain polar bonds. Select Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Leads to polar bonds within a molecule. If the diatomic molecule’s bond is polar, it is polar. Undqual sharing of bonding electron pairs is what. A polar molecule may have one or more lone pairs. The polarity of a molecule. If a molecule contains no dipole moments across any bonds it will be. There must be an odd number of. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved (1 point) Part 1 Which of the following statements Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Which of the following statements could be true regarding polar molecules? B) a molecule with symmetrically arranged polar bonds can be. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. There must be an odd number of polar bonds so that their polarities not cancel. A)there must be an. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.toppr.com
Which of the following statements is/are correct about SF4 molecule? Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If the diatomic molecule’s bond is polar, it is polar. The polarity of the molecule. B) a molecule with symmetrically arranged polar bonds can be. Leads to polar bonds within a molecule. Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED 23.Mark each of the following statements as either true (T) or Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices The polarity of a molecule. Lone (unshared) pairs of electrons on the central. Which of the following statements could be true regarding polar molecules? A polar molecule may have one or more lone pairs. If a molecule contains one polar bond, it is a polar molecule c. Polarity of molecule is defined as the the molecule is said to be. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Question 4 (1 point) Which of the following statements is false Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Undqual sharing of bonding electron pairs is what. If a molecule contains more than one polar bond, it is a polar molecule d. Leads to polar bonds within a molecule. Lone (unshared) pairs of electrons on the central. The polarity of the molecule. Which of the following statements is correct? If a molecule contains no dipole moments across any bonds. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED 6. Which of the following statements is INCORRECT? a. Polar Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Undqual sharing of bonding electron pairs is what. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. B) a molecule with symmetrically arranged polar bonds can be. If a molecule contains one polar bond, it is a polar molecule c. Leads to polar bonds within a molecule. The polarity. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Select all false statements about the below molecule Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains one polar bond, it is a polar molecule c. The polarity of a molecule. Which of the following statements is correct? Polarity of molecule is defined as the the molecule is said to be polar molecule when the centre of the positive charge are at the one side and the. There must be an odd number. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
VIDEO solution Among the following statements related to water Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices B) a molecule with symmetrically arranged polar bonds can be. The polarity of a molecule. If the diatomic molecule’s bond is polar, it is polar. The polarity of the molecule. Lone (unshared) pairs of electrons on the central. Leads to polar bonds within a molecule. Undqual sharing of bonding electron pairs is what. If a molecule contains no dipole moments. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Question 20 (2.5 points) Which of the following statements is Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Undqual sharing of bonding electron pairs is what. There must be an odd number of polar bonds so that their polarities not cancel. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. A polar molecule may have one or more lone pairs. Which of the following statements is correct?. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.chegg.com
Solved Part A The following molecules contain polar bonds. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices Leads to polar bonds within a molecule. Undqual sharing of bonding electron pairs is what. The polarity of a molecule. Lone (unshared) pairs of electrons on the central. There must be an odd number of polar bonds so that their polarities not cancel. A polar molecule may have one or more lone pairs. If a molecule contains no dipole moments. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.studocu.com
Activity Molecule polarity demonstration answers Molecule Polarity Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices A)there must be an odd number of polar bonds so that their polarities not cancel. Undqual sharing of bonding electron pairs is what. The polarity of the molecule. Which of the following statements could be true regarding polar molecules? B) a molecule with symmetrically arranged polar bonds can be. Leads to polar bonds within a molecule. There must be an. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED Consider a molecule of PBr3 and determine if each of the Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains more than one polar bond, it is a polar molecule d. Leads to polar bonds within a molecule. The polarity of the molecule. The polarity of a molecule. If a molecule contains one polar bond, it is a polar molecule c. If the diatomic molecule’s bond is polar, it is polar. A polar molecule may have. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVEDQuestion 5 pts Which of the following statements regarding Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices B) a molecule with symmetrically arranged polar bonds can be. A polar molecule may have one or more lone pairs. Undqual sharing of bonding electron pairs is what. The polarity of the molecule. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. If the diatomic molecule’s bond is polar,. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From www.numerade.com
SOLVED The following molecule contains two functional groups, a Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If a molecule contains one polar bond, it is a polar molecule c. There must be an odd number of polar bonds so that their polarities not cancel. Leads to polar bonds within a molecule. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Which of the following statements. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.
From brainly.com
Complete the following table. Tell if the molecule is polar or nonpolar Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices If the diatomic molecule’s bond is polar, it is polar. For a molecule that has polar bonds, the molecular geometry must be known in order to predict the overall polarity. Leads to polar bonds within a molecule. If a molecule contains more than one polar bond, it is a polar molecule d. There must be an odd number of polar. Which Of The Following Statements About The Polarity Of A Molecule Is False Group Of Answer Choices.