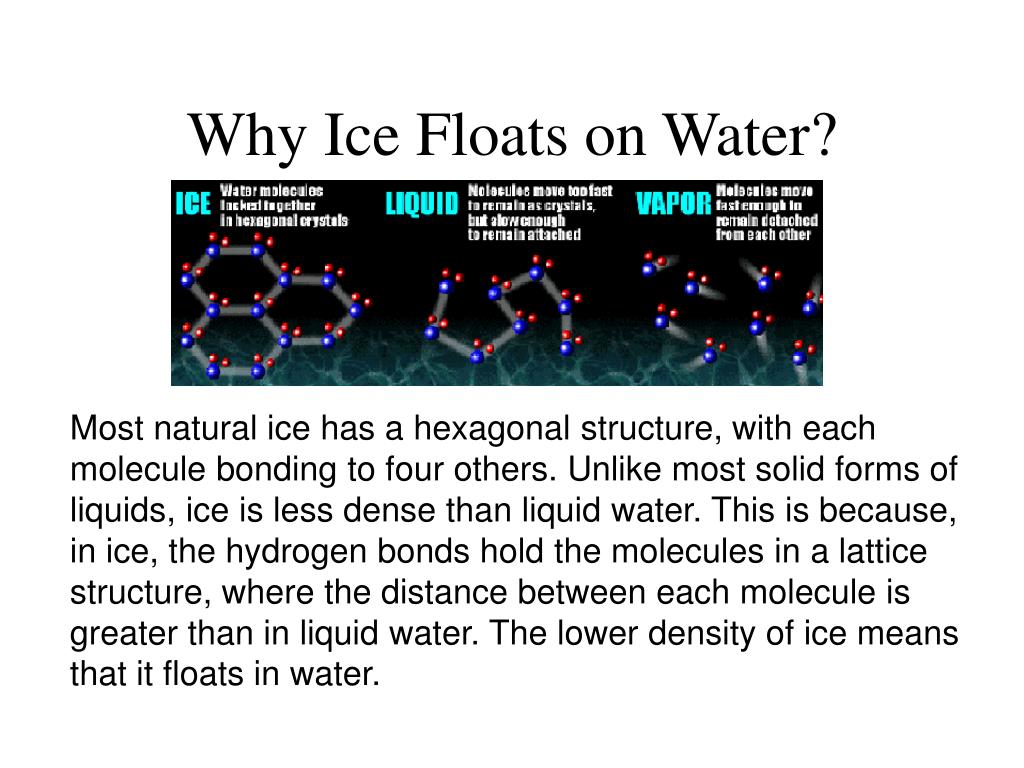
Why Does Ice Float On A Molecular Level . When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice floats because it is about 9% less dense than liquid water. Ponds or lakes begin to freeze at the surface, closer to the cold air. In other words, ice takes up about 9% more space than. This is because the molecules in ice are further apart. Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. All of this physics comes. Water is less dense in its frozen form, ice, than it is in its liquid form. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water.
from www.slideserve.com
In other words, ice takes up about 9% more space than. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice floats because it is about 9% less dense than liquid water. The hexagonal channels become partially filled, and the volume. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. Ponds or lakes begin to freeze at the surface, closer to the cold air. This is because the molecules in ice are further apart. All of this physics comes.
PPT C H A P T E R 11 Fluids PowerPoint Presentation, free download
Why Does Ice Float On A Molecular Level Water is less dense in its frozen form, ice, than it is in its liquid form. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than. The hexagonal channels become partially filled, and the volume. Ice is less dense than liquid water and so it floats. All of this physics comes. This is because the molecules in ice are further apart. Ponds or lakes begin to freeze at the surface, closer to the cold air. Water is less dense in its frozen form, ice, than it is in its liquid form.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level All of this physics comes. In other words, ice takes up about 9% more space than. This is because the molecules in ice are further apart. Ponds or lakes begin to freeze at the surface, closer to the cold air. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less. Why Does Ice Float On A Molecular Level.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float On A Molecular Level Water is less dense in its frozen form, ice, than it is in its liquid form. In other words, ice takes up about 9% more space than. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. Ponds or lakes begin to. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. Ice is less dense than liquid water and so it floats. Water is less dense in its frozen form, ice, than it is in its liquid form. In other words, ice takes up about 9% more space than. When ice melts, some of the hydrogen bonds. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT WHY? PowerPoint Presentation, free download ID2511404 Why Does Ice Float On A Molecular Level Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than. All of this physics comes. Water is less dense in its frozen form, ice, than it is in its liquid form. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses. Why Does Ice Float On A Molecular Level.
From www.youtube.com
Why Does Ice Float?!? Intermolecular Forces Explained Hydrogen Bonding Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ice is less dense than liquid water and so it floats. Water is less dense in its frozen form, ice, than it. Why Does Ice Float On A Molecular Level.
From slideplayer.com
Water Unit. ppt download Why Does Ice Float On A Molecular Level In other words, ice takes up about 9% more space than. Water is less dense in its frozen form, ice, than it is in its liquid form. All of this physics comes. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. The hexagonal channels become. Why Does Ice Float On A Molecular Level.
From www.youtube.com
Why ice floats on water Class 9 Chapter Chemistry NCERT YouTube Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ponds or lakes begin to freeze at the surface, closer to the cold air. All of this physics comes. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Show the Arrangement of Particles in Solid, Liquid and Gaseous Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. Ice is less dense than liquid water and so it floats. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. In other words, ice takes up about 9% more space than.. Why Does Ice Float On A Molecular Level.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. This is because the molecules in ice are further apart. Ponds or lakes begin to freeze at the surface, closer to the cold air. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice is less dense than liquid water and so it floats.. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Summary on intermolecular forces PowerPoint Presentation, free Why Does Ice Float On A Molecular Level Ice is less dense than liquid water and so it floats. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. This is because the molecules in ice are further apart. The hexagonal channels become partially filled, and the volume. When ice melts, some of the. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT C H A P T E R 11 Fluids PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level Ice floats because it is about 9% less dense than liquid water. This is because the molecules in ice are further apart. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ponds or lakes begin to freeze at the surface, closer to the cold air. The hexagonal channels become partially filled, and. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Chapter 17 Water and Aqueous Systems PowerPoint Presentation Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. Ponds or lakes begin to freeze at the surface, closer to the cold air. In other words, ice takes up about 9% more space than. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. So, the buoyant force will balance out the force. Why Does Ice Float On A Molecular Level.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Does Ice Float On A Molecular Level In other words, ice takes up about 9% more space than. This is because the molecules in ice are further apart. All of this physics comes. Ice floats because it is about 9% less dense than liquid water. Ice is less dense than liquid water and so it floats. When ice melts, some of the hydrogen bonds are broken and. Why Does Ice Float On A Molecular Level.
From www.pinterest.com
Water Expansion When Freezing Science Facts Water molecule Why Does Ice Float On A Molecular Level In other words, ice takes up about 9% more space than. Ice is less dense than liquid water and so it floats. All of this physics comes. The hexagonal channels become partially filled, and the volume. Ponds or lakes begin to freeze at the surface, closer to the cold air. Water is less dense in its frozen form, ice, than. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID9145406 Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. This is because the molecules in ice are further apart. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. Ponds or lakes begin to freeze at the surface, closer to the cold. Why Does Ice Float On A Molecular Level.
From www.youtube.com
Why ice floats on water??//class9//chemistry YouTube Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than. Ponds or lakes begin to freeze at the surface, closer to the cold air. So, the buoyant force will balance out the force of gravity if the density. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice is less dense than liquid water and so it floats. Ice floats. Why Does Ice Float On A Molecular Level.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. All of this physics comes. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice is less dense than liquid water and so it floats. The hexagonal channels become. Why Does Ice Float On A Molecular Level.
From youngzine.org
What Makes Ice Slippery? Youngzine Science Why Does Ice Float On A Molecular Level Water is less dense in its frozen form, ice, than it is in its liquid form. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ponds or lakes. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Polar Bonds and Molecules PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. All of this physics comes. Ice floats because it is about 9% less dense than liquid water. Ponds or. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Polar Bonds and Molecules PowerPoint Presentation, free download Why Does Ice Float On A Molecular Level When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hexagonal channels become partially filled, and the volume. Ponds or lakes begin to freeze at the surface, closer to the cold air. In other words, ice takes up about 9% more space than. This is because the molecules in ice are further. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Solids differ Hardness Melting point Flexibility Conductivity Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. In other words, ice takes up about 9% more space than. This is because the molecules in ice are further apart. Ponds or lakes begin to freeze at the surface, closer to the cold air. All of this physics comes. Ice floats because it is about 9% less dense than liquid. Why Does Ice Float On A Molecular Level.
From tinyhousegarage.com
Why Does Ice Float On Water Chemistry? Why Does Ice Float On A Molecular Level In other words, ice takes up about 9% more space than. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. Ice floats because it is. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID2718491 Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ice floats because it is about 9% less dense than liquid water. This is because the molecules in ice are further apart.. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Summary on intermolecular forces PowerPoint Presentation, free Why Does Ice Float On A Molecular Level Water is less dense in its frozen form, ice, than it is in its liquid form. All of this physics comes. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hexagonal channels become partially filled, and the volume. Ice floats because it is about 9% less dense than liquid water. Ponds. Why Does Ice Float On A Molecular Level.
From issuu.com
Why Does Ice Float On Water Chemistry? by AmericanLifeguardManual Issuu Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ice floats because it is about 9% less dense than liquid water. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hexagonal channels become partially filled, and. Why Does Ice Float On A Molecular Level.
From www.pinterest.com
Why does ice float in water? Zaidan and Charles Morton Why Does Ice Float On A Molecular Level Water is less dense in its frozen form, ice, than it is in its liquid form. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. All of this physics comes. Ponds or lakes begin to freeze at the surface, closer to the cold air. Ice is less dense than liquid water and. Why Does Ice Float On A Molecular Level.
From answerdbmaligner.z21.web.core.windows.net
Why Can Ice Float On Water Why Does Ice Float On A Molecular Level The hexagonal channels become partially filled, and the volume. Ice floats because it is about 9% less dense than liquid water. Ice is less dense than liquid water and so it floats. Water is less dense in its frozen form, ice, than it is in its liquid form. So, the buoyant force will balance out the force of gravity if. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. This is because the molecules in ice are further apart. Water is less dense in its frozen form, ice, than it is in its liquid form. So, the buoyant force will balance out the force of gravity if the density of the object is less than. Why Does Ice Float On A Molecular Level.
From byjus.com
why does ice float on wate Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Water is less dense in its frozen form, ice, than it is in its liquid form. In other words, ice takes up about 9% more space than. Ponds or lakes begin to freeze at the surface,. Why Does Ice Float On A Molecular Level.
From www.livescience.com
Why does ice float? Live Science Why Does Ice Float On A Molecular Level In other words, ice takes up about 9% more space than. All of this physics comes. This is because the molecules in ice are further apart. Ice floats because it is about 9% less dense than liquid water. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. So, the buoyant force will. Why Does Ice Float On A Molecular Level.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Why Does Ice Float On A Molecular Level Ice is less dense than liquid water and so it floats. All of this physics comes. The hexagonal channels become partially filled, and the volume. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. In other words, ice takes up about 9% more space than. Ice floats because it is about 9%. Why Does Ice Float On A Molecular Level.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Water is less dense in its frozen form, ice, than it is in its liquid form. Ice is less dense than liquid water and so it floats. Ice floats because it is about 9% less dense. Why Does Ice Float On A Molecular Level.
From www.numerade.com
SOLVED Explain clearly why ice floats on liquid water. Make sure your Why Does Ice Float On A Molecular Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. The hexagonal channels become partially filled, and the volume. This is because the molecules in ice are further apart. Ice is less dense than liquid water and so it floats. All of this physics comes. Water. Why Does Ice Float On A Molecular Level.
From www.showme.com
Why does ice float? ShowMe Why Does Ice Float On A Molecular Level Ponds or lakes begin to freeze at the surface, closer to the cold air. Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. Water is less dense in its frozen form, ice, than it is in its liquid form. In other words, ice takes up about 9% more space. Why Does Ice Float On A Molecular Level.