Serial Dilution Definition Quizlet . A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. The purpose of a serial dilution is to estimate the concentration of a. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step.
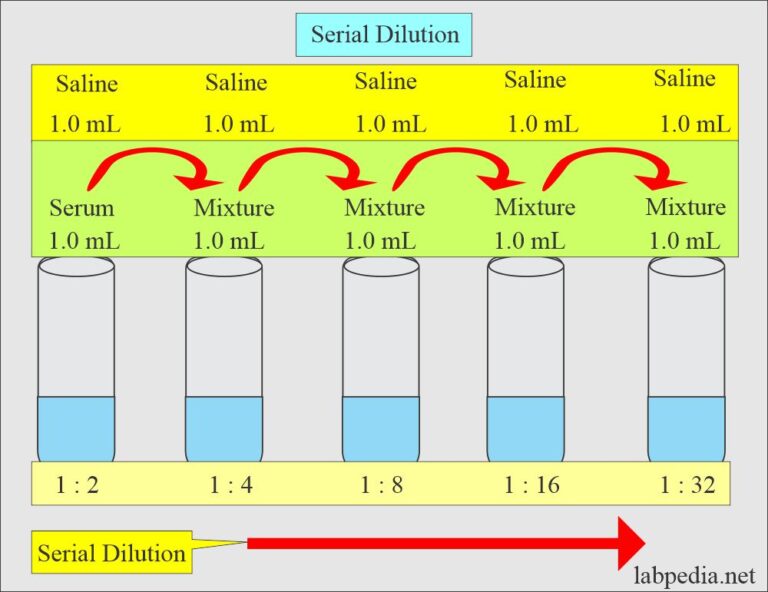
from labpedia.net
Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. The purpose of a serial dilution is to estimate the concentration of a. The concentration factor is the initial volume divided by the final solution volume. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step.
Solutions Part 1 Solutions Preparation used in Clinical Laboratory
Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and. Serial Dilution Definition Quizlet.
From www.shutterstock.com
Serial Dilution Stepwise Dilution Substance Solution Stock Vector Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorise flashcards containing terms like how. Serial Dilution Definition Quizlet.
From www.medicine.mcgill.ca
Serial Dilutions Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions. Serial Dilution Definition Quizlet.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The concentration factor is the initial volume divided by the final solution volume. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as. Serial Dilution Definition Quizlet.
From www.chegg.com
Solved 1. Look at the serial dilution image above. If your Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a series of dilutions, with the dilution factor staying the same. Serial Dilution Definition Quizlet.
From www.slideshare.net
Serial dilution Serial Dilution Definition Quizlet Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A. Serial Dilution Definition Quizlet.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The purpose of a serial dilution is to. Serial Dilution Definition Quizlet.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The purpose of a serial dilution is. Serial Dilution Definition Quizlet.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorise flashcards containing terms like. Serial Dilution Definition Quizlet.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. Study with quizlet and memorise flashcards containing terms like how do you measure the growth. Serial Dilution Definition Quizlet.
From www.freepik.com
Premium Vector Serial Dilutions science vector illustration infographic Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? Study with quizlet and memorize flashcards containing. Serial Dilution Definition Quizlet.
From iul-instruments.com
Serial dilution techniques and requirements IUL Instruments Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. Study with quizlet and memorise flashcards containing terms like. Serial Dilution Definition Quizlet.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? Study with quizlet and memorize flashcards containing. Serial Dilution Definition Quizlet.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Serial Dilution Definition Quizlet Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing. Serial Dilution Definition Quizlet.
From microbialnotes.com
Serial dilution how do we prepare it? Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? Study with quizlet and memorize flashcards containing terms like dilution, by performing series. Serial Dilution Definition Quizlet.
From mungfali.com
10 Fold Serial Dilution Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as. Serial Dilution Definition Quizlet.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as. Serial Dilution Definition Quizlet.
From www.youtube.com
Serial Dilutions Microbiology YouTube Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions. Serial Dilution Definition Quizlet.
From www.chegg.com
Solved Perform 5 15 serial dilutions; 1 mL final volume. Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The concentration. Serial Dilution Definition Quizlet.
From www.thetechedvocate.org
How to Calculate Serial Dilutions The Tech Edvocate Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorise flashcards containing terms like how. Serial Dilution Definition Quizlet.
From www.biorender.com
Serial Dilution Procedure BioRender Science Templates Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorize flashcards containing terms like dilution,. Serial Dilution Definition Quizlet.
From www.youtube.com
How to Calculate CFUSerial Dilution Microbiology Technique knowledge Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorize flashcards containing terms like dilution,. Serial Dilution Definition Quizlet.
From www.youtube.com
How to Perform Serial Dilution for Bacterial Growth Measurement Step Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. Study with quizlet and memorise flashcards containing terms like how. Serial Dilution Definition Quizlet.
From www.youtube.com
Serial Dilution Calculations Examples YouTube Serial Dilution Definition Quizlet A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is any dilution in which the concentration decreases by the same factor in. Serial Dilution Definition Quizlet.
From study.com
Serial Dilution Definition, Purpose & Calculation Lesson Serial Dilution Definition Quizlet A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is any dilution in which the concentration. Serial Dilution Definition Quizlet.
From www.integra-biosciences.com
Guide Learn how to perform serial dilutions with INTEGRA INTEGRA Serial Dilution Definition Quizlet A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is any dilution in which the concentration decreases by the same factor. Serial Dilution Definition Quizlet.
From mungfali.com
Serial Dilution Steps Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is any dilution in which the concentration decreases by the same factor. Serial Dilution Definition Quizlet.
From thestudentnotes.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses The Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? The concentration factor is the initial volume divided by the final solution volume. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. A serial dilution is a set of. Serial Dilution Definition Quizlet.
From www.youtube.com
Serial Dilution Calculations YouTube Serial Dilution Definition Quizlet The concentration factor is the initial volume divided by the final solution volume. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as. Serial Dilution Definition Quizlet.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. Study with quizlet and memorize flashcards containing. Serial Dilution Definition Quizlet.
From www.learnsci.com
LearnSci Smart Worksheet Serial Dilutions Serial Dilution Definition Quizlet Study with quizlet and memorize flashcards containing terms like dilution, by performing series of dilutions (serial dilution), little, correct. A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing. Serial Dilution Definition Quizlet.
From www.chegg.com
Solved You perform a serial dilution as shown in the figure Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a series of dilutions, with the dilution factor staying the same for each step. A serial dilution is any dilution in which. Serial Dilution Definition Quizlet.
From www.youtube.com
Serial Dilution Required Practical Revision for Biology and Chemistry Serial Dilution Definition Quizlet Study with quizlet and memorise flashcards containing terms like how do you measure the growth of the bacteria in the culture? A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. A serial dilution is a series of. Serial Dilution Definition Quizlet.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Serial Dilution Definition Quizlet A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. A serial dilution is a set of dilutions of a stock standard, stock reagent, or body fluid specimen that is prepared by utilizing each dilution as the starting solution for the next. The purpose of a serial dilution is to estimate. Serial Dilution Definition Quizlet.
From www.reddit.com
Serial Dilution in Microbiology Definition, Formula, Procedure and Serial Dilution Definition Quizlet The purpose of a serial dilution is to estimate the concentration of a. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. The concentration factor is the initial volume divided by the final solution volume. A serial dilution is a set of dilutions of a stock standard, stock reagent, or. Serial Dilution Definition Quizlet.