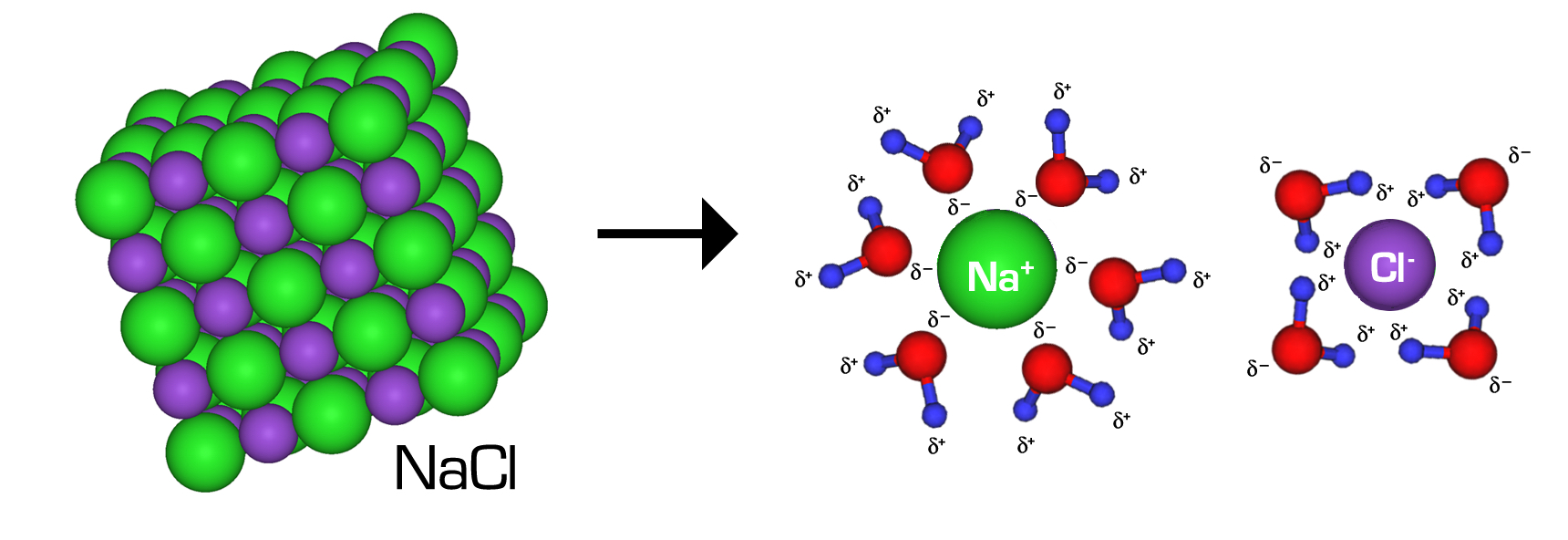
Nac1 (Table Salt) Dissolves In Water . Study with quizlet and memorize flashcards containing terms like 1. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Substances that dissolve in water to yield ions are called electrolytes. When salt is added to water, the positive and negative ions separate. Nacl (table salt) dissolves in water., 2. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? The water molecules surround the ions and pull them. Nonelectrolytes are substances that do not. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it.
from visionlearning.com
When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: The water molecules surround the ions and pull them. Study with quizlet and memorize flashcards containing terms like 1. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nacl (table salt) dissolves in water., 2. Substances that dissolve in water to yield ions are called electrolytes. When salt is added to water, the positive and negative ions separate. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nonelectrolytes are substances that do not. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it.
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning
Nac1 (Table Salt) Dissolves In Water When salt is added to water, the positive and negative ions separate. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: When salt is added to water, the positive and negative ions separate. Study with quizlet and memorize flashcards containing terms like 1. The water molecules surround the ions and pull them. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Nonelectrolytes are substances that do not. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Substances that dissolve in water to yield ions are called electrolytes. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nacl (table salt) dissolves in water., 2.
From www.youtube.com
Table Salt (NaCl) and Its Dissolving in Water Under a Microscope (40x1000x) YouTube Nac1 (Table Salt) Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Well, a chemical change involves a chemical reaction, with new substances. Nac1 (Table Salt) Dissolves In Water.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. Study with quizlet and memorize flashcards containing terms like 1. Nacl (table salt) dissolves in water., 2. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Substances that dissolve in water to yield ions are called electrolytes. Well, a chemical change involves a chemical reaction, with new substances. Nac1 (Table Salt) Dissolves In Water.
From mungfali.com
Dissolving Salt In Water Diagram Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nacl (table salt) dissolves in water., 2. When salt is added to water, the positive and negative ions separate. When you dissolve $\ce{nh3}$ in water, the following equilibrium. Nac1 (Table Salt) Dissolves In Water.
From slidetodoc.com
Salts and Solubility Activity 3 Solution Equilibrium and Nac1 (Table Salt) Dissolves In Water Study with quizlet and memorize flashcards containing terms like 1. Nonelectrolytes are substances that do not. Nacl (table salt) dissolves in water., 2. The water molecules surround the ions and pull them. Substances that dissolve in water to yield ions are called electrolytes. Imagine that you have a flask filled with water, and a selection of substances that you will. Nac1 (Table Salt) Dissolves In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube Nac1 (Table Salt) Dissolves In Water Study with quizlet and memorize flashcards containing terms like 1. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nonelectrolytes are substances that do not. Substances that dissolve. Nac1 (Table Salt) Dissolves In Water.
From users.highland.edu
Aqueous Solutions Nac1 (Table Salt) Dissolves In Water Nacl (table salt) dissolves in water., 2. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Study with quizlet and memorize flashcards containing terms like 1. When salt is added to. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Chapter 10 Chemical Reactivity PowerPoint Presentation, free download ID2068466 Nac1 (Table Salt) Dissolves In Water When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. Study with quizlet and memorize flashcards containing terms like 1. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nonelectrolytes are substances that do not. Imagine that you have a. Nac1 (Table Salt) Dissolves In Water.
From www.numerade.com
SOLVED 7 How do the properties of a solution made with a strong electrolyte (such as NaCl Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. Nonelectrolytes are substances that do not. Nacl (table salt) dissolves in water., 2. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Substances that dissolve in water to yield ions are called electrolytes. When you dissolve $\ce{nh3}$ in water, the following equilibrium. Nac1 (Table Salt) Dissolves In Water.
From masterconceptsinchemistry.com
How does sodium chloride (NaCl) dissolve in water? Nac1 (Table Salt) Dissolves In Water Study with quizlet and memorize flashcards containing terms like 1. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. The water molecules surround the ions and pull them. Nonelectrolytes are substances that do not. When salt is added to water, the positive and negative ions separate. Substances that dissolve in water. Nac1 (Table Salt) Dissolves In Water.
From courses.lumenlearning.com
Water Introduction to Biology Nac1 (Table Salt) Dissolves In Water Nonelectrolytes are substances that do not. Study with quizlet and memorize flashcards containing terms like 1. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. The water molecules surround the ions and pull them. Well, a chemical change involves a chemical reaction, with. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free download ID1242210 Nac1 (Table Salt) Dissolves In Water Substances that dissolve in water to yield ions are called electrolytes. The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. When you dissolve table salt (sodium chloride, also known as. Nac1 (Table Salt) Dissolves In Water.
From geoverse.co.uk
Geoverse Poems on geology, science, Horsham, life, the universe and everything Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. Study with quizlet and memorize flashcards containing terms like 1. Substances that dissolve in water to yield ions are called electrolytes. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nacl (table salt) dissolves in water., 2. Imagine that you have a. Nac1 (Table Salt) Dissolves In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning Nac1 (Table Salt) Dissolves In Water Nonelectrolytes are substances that do not. Study with quizlet and memorize flashcards containing terms like 1. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. When salt is added to water, the positive and negative ions separate. When you dissolve table salt (sodium chloride, also known as nacl) in water, are. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Chemical Equations Review PowerPoint Presentation, free download ID3818238 Nac1 (Table Salt) Dissolves In Water When salt is added to water, the positive and negative ions separate. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Study with quizlet and memorize flashcards containing terms like 1. Nacl (table salt) dissolves in water., 2. The water molecules surround the ions and pull them. Nonelectrolytes are substances that do not. Imagine that you have. Nac1 (Table Salt) Dissolves In Water.
From exovcunbz.blob.core.windows.net
Nacl (Table Salt) Dissolves In Water at Ira Canup blog Nac1 (Table Salt) Dissolves In Water Nacl (table salt) dissolves in water., 2. Nonelectrolytes are substances that do not. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Study with quizlet and memorize flashcards containing terms like 1. When salt. Nac1 (Table Salt) Dissolves In Water.
From slideplayer.com
Chapter 4 Elements, Compounds, and Mixtures … Oh My! ppt download Nac1 (Table Salt) Dissolves In Water Substances that dissolve in water to yield ions are called electrolytes. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Nacl (table salt) dissolves in water., 2. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nonelectrolytes are substances that do not. Imagine that you have a flask. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID6136171 Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Nacl (table salt) dissolves in water., 2. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nonelectrolytes are substances that do not. Substances that dissolve. Nac1 (Table Salt) Dissolves In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Nac1 (Table Salt) Dissolves In Water Nacl (table salt) dissolves in water., 2. Substances that dissolve in water to yield ions are called electrolytes. The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how. Nac1 (Table Salt) Dissolves In Water.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Nac1 (Table Salt) Dissolves In Water Nacl (table salt) dissolves in water., 2. Nonelectrolytes are substances that do not. Study with quizlet and memorize flashcards containing terms like 1. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Imagine that you have. Nac1 (Table Salt) Dissolves In Water.
From mungfali.com
Dissolving Salt In Water Diagram Nac1 (Table Salt) Dissolves In Water When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: The water molecules surround the ions and pull them. Nonelectrolytes are substances that do not. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nacl (table salt) dissolves in water., 2. Study with quizlet and memorize flashcards containing terms. Nac1 (Table Salt) Dissolves In Water.
From shaunmwilliams.com
Chapter 11 Presentation Nac1 (Table Salt) Dissolves In Water Nacl (table salt) dissolves in water., 2. When salt is added to water, the positive and negative ions separate. Study with quizlet and memorize flashcards containing terms like 1. Substances that dissolve in water to yield ions are called electrolytes. The water molecules surround the ions and pull them. Well, a chemical change involves a chemical reaction, with new substances. Nac1 (Table Salt) Dissolves In Water.
From slideplayer.com
GLE 1 Both elements and compounds are pure substances ppt download Nac1 (Table Salt) Dissolves In Water Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Study with quizlet and memorize flashcards containing terms like 1. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. When you dissolve $\ce{nh3}$ in water,. Nac1 (Table Salt) Dissolves In Water.
From www.vecteezy.com
Water solubility of sodium chloride NaCl or salt. Aquous solution of hydrated cations and Nac1 (Table Salt) Dissolves In Water Study with quizlet and memorize flashcards containing terms like 1. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Nacl (table salt) dissolves in water., 2. Nonelectrolytes are substances that. Nac1 (Table Salt) Dissolves In Water.
From sciencenotes.org
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Nac1 (Table Salt) Dissolves In Water Substances that dissolve in water to yield ions are called electrolytes. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Well, a chemical change involves a chemical reaction, with new substances produced as a. Nac1 (Table Salt) Dissolves In Water.
From stock.adobe.com
Common table salt, sodium chloride (NaCl) and how it dissolves in water vector de Stock Adobe Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. Study with quizlet and memorize flashcards containing terms like 1. Nonelectrolytes are substances that do not. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Well, a chemical change involves a chemical reaction, with. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Macromolecules PowerPoint Presentation, free download ID5948789 Nac1 (Table Salt) Dissolves In Water When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Substances that dissolve in water to yield ions are called electrolytes. When you dissolve table salt (sodium chloride, also known as nacl). Nac1 (Table Salt) Dissolves In Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water Nac1 (Table Salt) Dissolves In Water When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Substances that dissolve in water to yield ions are called electrolytes. Study with quizlet and memorize flashcards containing terms like 1. When you dissolve table salt (sodium chloride, also known. Nac1 (Table Salt) Dissolves In Water.
From exovcunbz.blob.core.windows.net
Nacl (Table Salt) Dissolves In Water at Ira Canup blog Nac1 (Table Salt) Dissolves In Water Substances that dissolve in water to yield ions are called electrolytes. When salt is added to water, the positive and negative ions separate. Nacl (table salt) dissolves in water., 2. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. When you dissolve table salt (sodium chloride, also known as nacl) in. Nac1 (Table Salt) Dissolves In Water.
From www.youtube.com
table salt dissolves in water YouTube Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Substances that dissolve in water to yield ions are called. Nac1 (Table Salt) Dissolves In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2736092 Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nonelectrolytes are substances that do not. When you dissolve $\ce{nh3}$ in water, the following equilibrium is. Nac1 (Table Salt) Dissolves In Water.
From www.youtube.com
table salt dissolves in water YouTube Nac1 (Table Salt) Dissolves In Water Substances that dissolve in water to yield ions are called electrolytes. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Nonelectrolytes are substances that do not. Nacl (table salt) dissolves in water., 2. Well, a chemical change involves a chemical reaction, with new. Nac1 (Table Salt) Dissolves In Water.
From exovcunbz.blob.core.windows.net
Nacl (Table Salt) Dissolves In Water at Ira Canup blog Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Substances that dissolve in water to yield ions are called electrolytes. Nacl (table salt) dissolves in water., 2. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: When you dissolve table salt (sodium chloride, also known. Nac1 (Table Salt) Dissolves In Water.
From www.chegg.com
Solved 5. Show NaCl and label them with the correct charge Nac1 (Table Salt) Dissolves In Water Nonelectrolytes are substances that do not. Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in it. Study with quizlet and memorize flashcards containing terms like 1.. Nac1 (Table Salt) Dissolves In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Nac1 (Table Salt) Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? The water molecules surround the ions and pull them. Nacl (table salt) dissolves in water., 2. Nonelectrolytes are substances that do not. Substances that dissolve in water to yield ions are called electrolytes. Well, a chemical change. Nac1 (Table Salt) Dissolves In Water.
From www.chegg.com
Solved You notice that NaCl salt easily solubles in water, Nac1 (Table Salt) Dissolves In Water The water molecules surround the ions and pull them. When you dissolve $\ce{nh3}$ in water, the following equilibrium is set up: When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nonelectrolytes are substances that do not. Study with quizlet and memorize flashcards containing terms like 1.. Nac1 (Table Salt) Dissolves In Water.