Titration Of Hcl With Naoh Procedure . Hcl + naoh → nacl + h 2 o. Volume measurements play a key role in titration. In equation 1, the acid is hcl. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Perform and interpret titration calculations. The initial volume and final volume of all three trails were subtracted to find each amount of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Dilute with distilled water to about 100 ml. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. You have to decide if this experiment is suitable to use with different classes, and look at the need for.
from studylib.net
One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Hcl + naoh → nacl + h 2 o. The initial volume and final volume of all three trails were subtracted to find each amount of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Dilute with distilled water to about 100 ml. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Perform and interpret titration calculations.
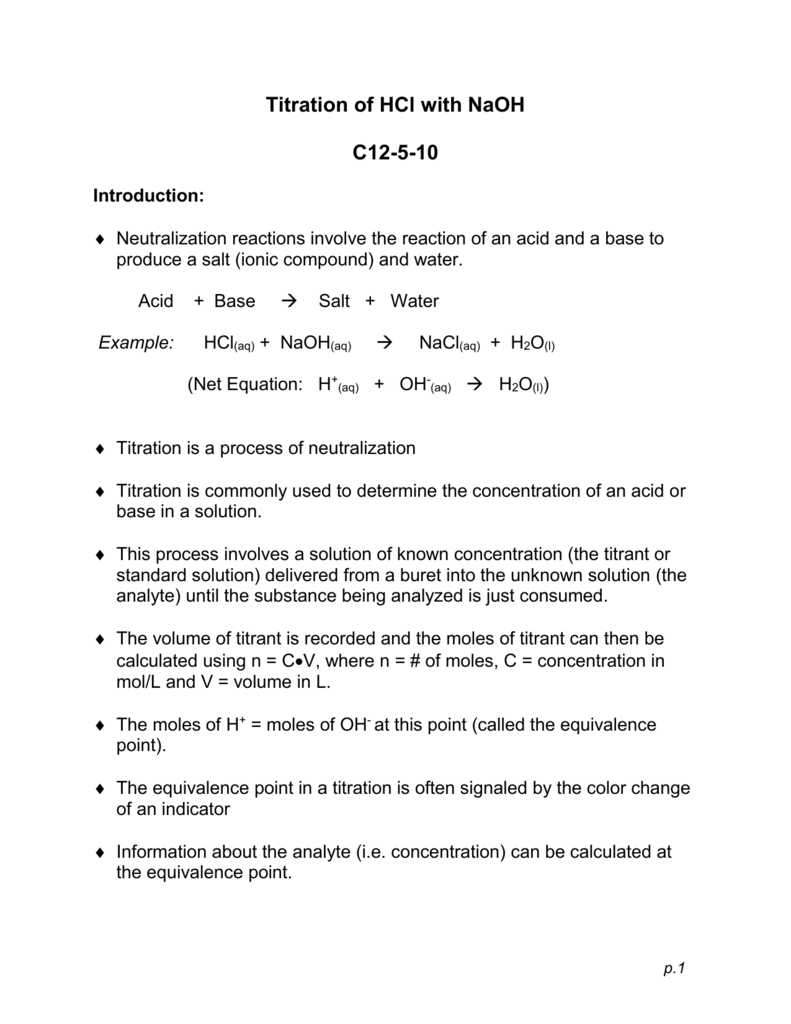
Titration of HCl with NaOH
Titration Of Hcl With Naoh Procedure One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements play a key role in titration. Dilute with distilled water to about 100 ml. The initial volume and final volume of all three trails were subtracted to find each amount of. Hcl + naoh → nacl + h 2 o. Perform and interpret titration calculations. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. In equation 1, the acid is hcl. You have to decide if this experiment is suitable to use with different classes, and look at the need for. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Of Hcl With Naoh Procedure The initial volume and final volume of all three trails were subtracted to find each amount of. Dilute with distilled water to about 100 ml. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration Of Hcl With Naoh Procedure.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Of Hcl With Naoh Procedure They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In equation 1, the acid is hcl. Dilute with distilled water to about 100 ml. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. The initial volume and final volume of all three trails were subtracted to find each amount of. One type of. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Hcl + naoh → nacl + h 2 o. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Dilute with distilled water to about 100 ml. Perform and interpret titration. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Dilute with distilled water to about 100 ml. Perform and. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the. Titration Of Hcl With Naoh Procedure.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Purpose The purpose of Titration Of Hcl With Naoh Procedure Volume measurements play a key role in titration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment students neutralise sodium hydroxide with hydrochloric. Titration Of Hcl With Naoh Procedure.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Of Hcl With Naoh Procedure Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Perform and interpret titration calculations. In equation 1, the acid is hcl. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Volume measurements play a key role in. Titration Of Hcl With Naoh Procedure.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Titration Of Hcl With Naoh Procedure They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Perform and interpret titration calculations. In equation 1, the acid is hcl. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: One type of titration uses a neutralization reaction, in which an acid. Titration Of Hcl With Naoh Procedure.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titration Of Hcl With Naoh Procedure Dilute with distilled water to about 100 ml. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure The initial volume and final volume of all three trails were subtracted to find each amount of. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as:. Titration Of Hcl With Naoh Procedure.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Chemistry Laboratory Titration Of Hcl With Naoh Procedure In equation 1, the acid is hcl. Dilute with distilled water to about 100 ml. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Since hcl and. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Volume measurements play a key role in titration. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. The initial volume and final. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure Perform and interpret titration calculations. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl. Dilute with distilled water to about 100 ml. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Perform and interpret titration calculations. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation. Titration Of Hcl With Naoh Procedure.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Of Hcl With Naoh Procedure Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Volume measurements play a key role in titration. Dilute with distilled water to about 100 ml. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. You have to decide if this experiment is suitable to use with different classes, and look at the need for. One type of titration. Titration Of Hcl With Naoh Procedure.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration with NaOH Titration Of Hcl With Naoh Procedure They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Hcl + naoh → nacl + h 2 o. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride. Titration Of Hcl With Naoh Procedure.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Of Hcl With Naoh Procedure Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Volume measurements play a key role in titration. The initial volume and final volume of all three trails were subtracted to find each amount of. In equation 1, the acid is hcl. Dilute with distilled water to about 100 ml. Perform and interpret titration calculations. You have to decide if. Titration Of Hcl With Naoh Procedure.
From mungfali.com
Acid Base Titration Procedure Titration Of Hcl With Naoh Procedure In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Perform and interpret titration calculations. Hcl + naoh → nacl +. Titration Of Hcl With Naoh Procedure.
From mavink.com
Titration Reaction Titration Of Hcl With Naoh Procedure One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A titration is a laboratory. Titration Of Hcl With Naoh Procedure.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Titration Of Hcl With Naoh Procedure Perform and interpret titration calculations. Volume measurements play a key role in titration. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Hcl + naoh → nacl + h 2. Titration Of Hcl With Naoh Procedure.
From www.numerade.com
SOLVED Write the laboratory report for determination of NaOH concentration by titration with Titration Of Hcl With Naoh Procedure Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Perform and interpret titration calculations. In equation 1, the acid is hcl. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Pipette aliquot of. Titration Of Hcl With Naoh Procedure.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Of Hcl With Naoh Procedure They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Dilute with distilled water to about 100 ml. Volume measurements play a key role in titration. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a. Titration Of Hcl With Naoh Procedure.
From millyvanilli-jan.weebly.com
Titration of HCL with Naoh Milly's Portfolio Titration Of Hcl With Naoh Procedure You have to decide if this experiment is suitable to use with different classes, and look at the need for. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: A titration is a laboratory technique used to. Titration Of Hcl With Naoh Procedure.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Titration Of Hcl With Naoh Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Dilute with distilled water to about 100 ml. Hcl + naoh → nacl + h 2 o. Volume measurements play a key role in titration. In equation 1, the acid is hcl. Since hcl and naoh fully dissociate into their ion. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure Perform and interpret titration calculations. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Dilute with distilled water to about 100 ml. Since hcl and naoh fully dissociate. Titration Of Hcl With Naoh Procedure.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Of Hcl With Naoh Procedure In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Perform and interpret titration calculations. Hcl + naoh → nacl + h 2 o. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Volume measurements play a key role. Titration Of Hcl With Naoh Procedure.
From studylib.net
Titration of HCl with NaOH Titration Of Hcl With Naoh Procedure Dilute with distilled water to about 100 ml. The initial volume and final volume of all three trails were subtracted to find each amount of. Hcl + naoh → nacl + h 2 o. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Hydrochloric acid reacts with sodium. Titration Of Hcl With Naoh Procedure.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the titration curve (pH versus Titration Of Hcl With Naoh Procedure You have to decide if this experiment is suitable to use with different classes, and look at the need for. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. The initial volume and final volume of all three trails were subtracted to find each amount of. In equation 1, the acid is hcl. Since hcl and naoh fully dissociate. Titration Of Hcl With Naoh Procedure.
From mungfali.com
HCl NaOH Titration Titration Of Hcl With Naoh Procedure In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Dilute with distilled water to about 100 ml. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A titration is a laboratory technique used to. Titration Of Hcl With Naoh Procedure.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Education Titration Of Hcl With Naoh Procedure Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Perform and interpret titration calculations. The initial volume and final volume of all three trails were subtracted to find each amount of. Dilute with distilled water to about 100 ml. Hcl + naoh → nacl + h 2 o.. Titration Of Hcl With Naoh Procedure.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Of Hcl With Naoh Procedure Hcl + naoh → nacl + h 2 o. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Volume measurements play a key role in titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration. Titration Of Hcl With Naoh Procedure.
From www.youtube.com
Tutorial Titration of Hydrochloric Acid and Sodium Hydroxide YouTube Titration Of Hcl With Naoh Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements play a key role in titration. You have to decide if this experiment is suitable to use. Titration Of Hcl With Naoh Procedure.
From www.scribd.com
Standardization of Hydrochloric Acid Sodium Carbonate Titration Titration Of Hcl With Naoh Procedure You have to decide if this experiment is suitable to use with different classes, and look at the need for. Volume measurements play a key role in titration. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. Hcl + naoh → nacl + h 2 o. In equation 1, the acid is hcl. One type of titration uses a. Titration Of Hcl With Naoh Procedure.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base (NaOH) YouTube Titration Of Hcl With Naoh Procedure Dilute with distilled water to about 100 ml. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find each amount of. Pipette aliquot of hydrochloric acid solution into 250ml erlenmeyer flask. One type of titration uses a neutralization reaction, in which. Titration Of Hcl With Naoh Procedure.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Of Hcl With Naoh Procedure Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The initial volume and. Titration Of Hcl With Naoh Procedure.