How Sodium Soap Is Prepared In The Laboratory . learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Test the properties of your soap. This fact gives soap its. Follow the procedure, report your. the reaction goes as (if using sodium hydroxide): learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment.
from www.alamy.com
learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. the reaction goes as (if using sodium hydroxide): learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. This fact gives soap its. Follow the procedure, report your. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. Test the properties of your soap.
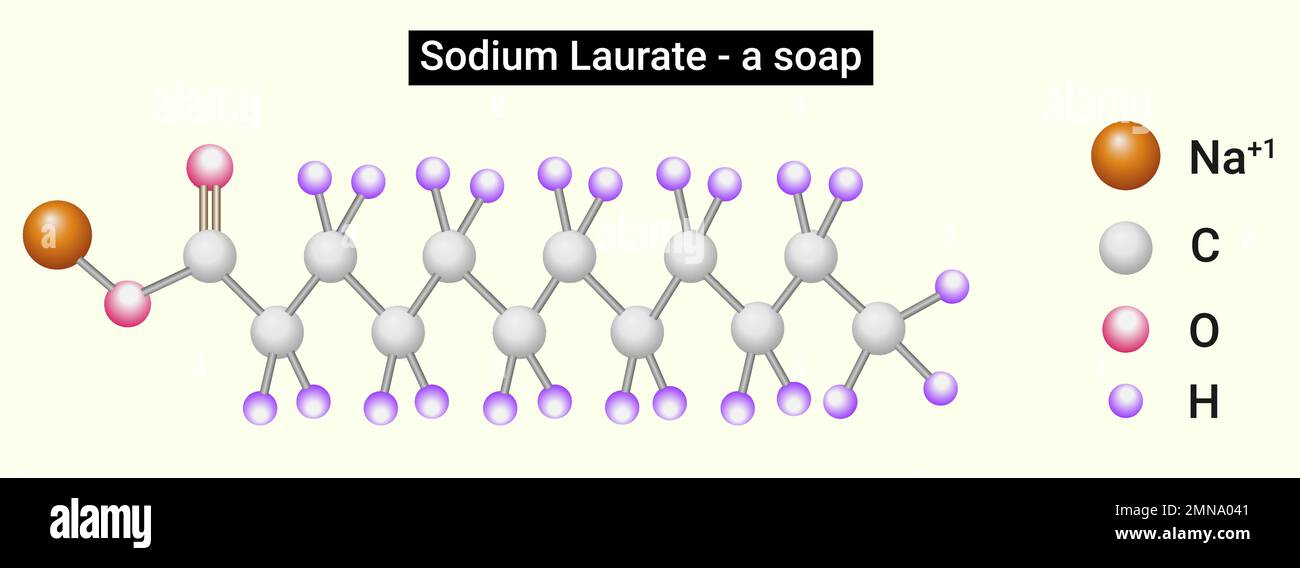
Structure of Sodium Laurate (soap Stock Vector Image & Art Alamy
How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. Test the properties of your soap. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. This fact gives soap its. learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. Follow the procedure, report your. the reaction goes as (if using sodium hydroxide):
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID1978873 How Sodium Soap Is Prepared In The Laboratory soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. the reaction goes as (if using sodium hydroxide): learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. learn how to make. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): Test the properties of your soap. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. soaps are the sodium and. How Sodium Soap Is Prepared In The Laboratory.
From edurev.in
Cleansing Action of Soaps and Detergents Class 10 Notes EduRev How Sodium Soap Is Prepared In The Laboratory Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. the reaction goes as (if using sodium hydroxide): One molecule of fat reacts with three molecules of sodium hydroxide to. How Sodium Soap Is Prepared In The Laboratory.
From questions-in.kunduz.com
A. Describe the texture for a sodium soap Organic Chemistry How Sodium Soap Is Prepared In The Laboratory learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. Follow the procedure, report your. the reaction goes as (if using sodium hydroxide): learn how soap is made by. How Sodium Soap Is Prepared In The Laboratory.
From www.youtube.com
Soap preparation in lab YouTube How Sodium Soap Is Prepared In The Laboratory learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. Test the properties of your soap. Preparation of soap soap is made of molecules that have one polar end. How Sodium Soap Is Prepared In The Laboratory.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. soaps are the sodium and potassium salts of long chain fatty acids that are. How Sodium Soap Is Prepared In The Laboratory.
From www.doubtnut.com
What is a 'soap' ? How is it prepared How Sodium Soap Is Prepared In The Laboratory This fact gives soap its. learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. Preparation. How Sodium Soap Is Prepared In The Laboratory.
From studylib.net
Saponification The preparation of Soap How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how soap is made by the saponification reaction, a. How Sodium Soap Is Prepared In The Laboratory.
From www.scribd.com
Properties of Sodium Soap PDF Sodium Hydroxide Surfactant How Sodium Soap Is Prepared In The Laboratory One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of. How Sodium Soap Is Prepared In The Laboratory.
From knowledgecycle.in
Foaming Capacity of Soaps Chemistry Investigatory Project PDF How Sodium Soap Is Prepared In The Laboratory This fact gives soap its. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how to make soap from fats or oils by heating them with sodium. How Sodium Soap Is Prepared In The Laboratory.
From www.alamy.com
Soaps are sodium or potassium salts of fatty acids which are formed by How Sodium Soap Is Prepared In The Laboratory learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. This fact gives soap its. . How Sodium Soap Is Prepared In The Laboratory.
From learnphysics-dhruv.blogspot.com
Physics Learn To prepare Soap by cold process. Science practical GSEB How Sodium Soap Is Prepared In The Laboratory learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. Follow the procedure, report your. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. One molecule of fat reacts with three molecules of sodium. How Sodium Soap Is Prepared In The Laboratory.
From www.slideshare.net
Chemistry of soaps How Sodium Soap Is Prepared In The Laboratory learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. This fact gives soap its. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Preparation of soap soap is made of molecules that have one. How Sodium Soap Is Prepared In The Laboratory.
From fineartamerica.com
Sodium Palmitate Soap Molecule Photograph by Molekuul/science Photo Library How Sodium Soap Is Prepared In The Laboratory soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Follow the procedure, report your. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. learn how to make soap from fats or oils by heating. How Sodium Soap Is Prepared In The Laboratory.
From www.youtube.com
Sodium Palmate from Soap YouTube How Sodium Soap Is Prepared In The Laboratory learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. Follow the procedure, report your. This fact gives soap its. soaps are the sodium and potassium salts of long chain fatty acids that. How Sodium Soap Is Prepared In The Laboratory.
From www.pinterest.com
How to Use Sodium Lactate Lactation, Sodium, Handmade soap How Sodium Soap Is Prepared In The Laboratory soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. the. How Sodium Soap Is Prepared In The Laboratory.
From www.scribd.com
Preparation of Soap Soap Sodium Hydroxide How Sodium Soap Is Prepared In The Laboratory This fact gives soap its. Follow the procedure, report your. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how soap is made by. How Sodium Soap Is Prepared In The Laboratory.
From www.alamy.com
Sodium palmitate soap molecule. Prepared from palm oil by How Sodium Soap Is Prepared In The Laboratory Follow the procedure, report your. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how to make soap by combining fat or. How Sodium Soap Is Prepared In The Laboratory.
From www.youtube.com
Chemistry Experiment How To Make Sodium Soap Full HD video YouTube How Sodium Soap Is Prepared In The Laboratory learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. Follow the procedure, report your. Test the properties of your soap. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. the reaction goes as (if using sodium hydroxide): soaps are the sodium. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 How Sodium Soap Is Prepared In The Laboratory Test the properties of your soap. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. This fact gives soap its. learn how to make soap from fats or oils by heating them with sodium hydroxide. How Sodium Soap Is Prepared In The Laboratory.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project How Sodium Soap Is Prepared In The Laboratory learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. This fact gives soap its. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance. How Sodium Soap Is Prepared In The Laboratory.
From peacecommission.kdsg.gov.ng
Sodium Soap How Sodium Soap Is Prepared In The Laboratory learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. Test the properties of your soap. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification. How Sodium Soap Is Prepared In The Laboratory.
From www.alamy.com
Sodium palmitate soap molecule. Prepared from palm oil by How Sodium Soap Is Prepared In The Laboratory Follow the procedure, report your. This fact gives soap its. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. the reaction goes as (if using sodium hydroxide): learn how to make soap by combining fat or oil with sodium hydroxide, a process called. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT Sodium PowerPoint Presentation, free download ID5244898 How Sodium Soap Is Prepared In The Laboratory learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. This fact gives soap its. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide,. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID How Sodium Soap Is Prepared In The Laboratory Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how to make soap by combining fat or oil with sodium hydroxide, a process called saponification. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. the reaction goes. How Sodium Soap Is Prepared In The Laboratory.
From www.scribd.com
Lab Report Soap Making Soap Sodium Hydroxide How Sodium Soap Is Prepared In The Laboratory Follow the procedure, report your. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Preparation of soap soap is made of molecules that have one polar end and one. How Sodium Soap Is Prepared In The Laboratory.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry How Sodium Soap Is Prepared In The Laboratory One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID How Sodium Soap Is Prepared In The Laboratory Follow the procedure, report your. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. Test the properties of your soap. learn how soap. How Sodium Soap Is Prepared In The Laboratory.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): Test the properties of your soap. Preparation of soap soap is made of molecules that have one polar end and one nonpolar end. learn how to make soap from a fat or an oil by heating it with sodium hydroxide in this chemistry experiment. Follow the procedure, report your. This. How Sodium Soap Is Prepared In The Laboratory.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID How Sodium Soap Is Prepared In The Laboratory soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. Test the properties of your soap. learn how to make lye soap via the saponification. How Sodium Soap Is Prepared In The Laboratory.
From www.scribd.com
Soap Soap Sodium Hydroxide How Sodium Soap Is Prepared In The Laboratory learn how to make lye soap via the saponification reaction using olive oil, sodium hydroxide, fragrance and coloring. Test the properties of your soap. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how soap is made by the saponification reaction, a. How Sodium Soap Is Prepared In The Laboratory.
From www.youtube.com
How to make soap in lab? making hard soap using NaOH (sodium Hydroxide How Sodium Soap Is Prepared In The Laboratory learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and.. How Sodium Soap Is Prepared In The Laboratory.
From www.scribd.com
soapmanufacturingprocess Soap Sodium Hydroxide How Sodium Soap Is Prepared In The Laboratory soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. learn how to make soap from fats or oils by heating them with sodium hydroxide in this wet lab experiment. learn how to make soap by combining fat or oil with sodium hydroxide, a. How Sodium Soap Is Prepared In The Laboratory.
From www.alamy.com
Structure of Sodium Laurate (soap Stock Vector Image & Art Alamy How Sodium Soap Is Prepared In The Laboratory One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. soaps are the sodium and potassium salts of long chain fatty acids that are generally made by saponification (alkaline hydrolysis) of natural fats,. Follow the procedure, report your. the reaction goes as (if using sodium hydroxide): learn how to make lye. How Sodium Soap Is Prepared In The Laboratory.
From www.researchgate.net
Figure 1 Flow diagram of soap production by bath process. Scientific How Sodium Soap Is Prepared In The Laboratory the reaction goes as (if using sodium hydroxide): One molecule of fat reacts with three molecules of sodium hydroxide to produce one glycerol and. learn how soap is made by the saponification reaction, a chemical process that involves fatty acids and alkali metals. learn how to make soap from a fat or an oil by heating it. How Sodium Soap Is Prepared In The Laboratory.