Flame Test Lab Answers Quizlet . What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? Flame tests lab flashcards | quizlet. How is it done?, under what. Indicate the specific energy change occurring in the ion. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. In this lab, you will perform flame tests of several different metal cations. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. Click the card to flip 👆. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: The flame tests for sodium sulfate and sodium chloride. Study with quizlet and memorize flashcards containing terms like what is a flame test? Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. Why does a metallic ion. Test the different metal salt solutions in a hot flame and.
from www.chegg.com
Study with quizlet and memorize flashcards containing terms like what is a flame test? What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. Click the card to flip 👆. In this lab, you will perform flame tests of several different metal cations. Test the different metal salt solutions in a hot flame and. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Flame tests lab flashcards | quizlet.
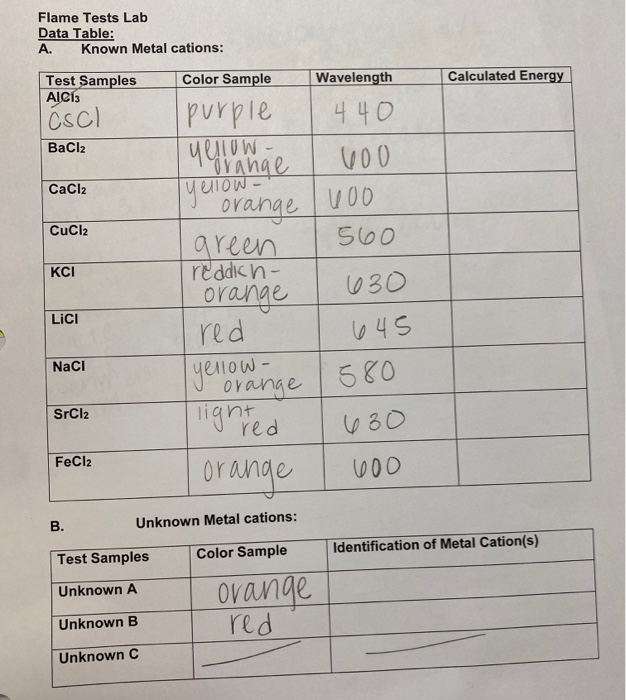
Solved Flame Tests Lab Data Table A. Known Metal cations
Flame Test Lab Answers Quizlet Indicate the specific energy change occurring in the ion. Test the different metal salt solutions in a hot flame and. Indicate the specific energy change occurring in the ion. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Why does a metallic ion. Click the card to flip 👆. Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. How is it done?, under what. To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: Flame tests lab flashcards | quizlet. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Study with quizlet and memorize flashcards containing terms like what is a flame test? The flame tests for sodium sulfate and sodium chloride. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? In this lab, you will perform flame tests of several different metal cations.
From myans.bhantedhammika.net
Atomic Emission And Flame Test Lab Answers Flame Test Lab Answers Quizlet The flame tests for sodium sulfate and sodium chloride. Flame tests lab flashcards | quizlet. Indicate the specific energy change occurring in the ion. How is it done?, under what. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. The lowest?, what are the electrons of each elements doing when you observe the colors. Flame Test Lab Answers Quizlet.
From studylib.net
Flame Test Exploration Flame Test Lab Answers Quizlet Flame tests lab flashcards | quizlet. Why does a metallic ion. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Since a specific energy is needed to radiate specific. Flame Test Lab Answers Quizlet.
From www.chegg.com
Solved Flame Tests Lab Data Table A. Known Metal cations Flame Test Lab Answers Quizlet Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Indicate. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Flame Test Lab Answer Key Chemistry › Athens Mutual Student Corner Flame Test Lab Answers Quizlet The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Indicate the specific energy change occurring in the ion. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? How is it done?, under what. What does a flame test indicate about the energy changes taking. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Report Answers Flame Test Lab Answers Quizlet How is it done?, under what. To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: Flame tests lab flashcards | quizlet. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Study with quizlet and memorize flashcards. Flame Test Lab Answers Quizlet.
From www.studocu.com
Flame Test Lab Report Prof. McLoud Flame Test Lab Report Flame Test Lab Answers Quizlet In this lab, you will perform flame tests of several different metal cations. Why does a metallic ion. How is it done?, under what. Test the different metal salt solutions in a hot flame and. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several.. Flame Test Lab Answers Quizlet.
From exyrnkwka.blob.core.windows.net
Flame Test Lab Chemistry Answers at Hannah Becker blog Flame Test Lab Answers Quizlet In this lab, you will perform flame tests of several different metal cations. Test the different metal salt solutions in a hot flame and. Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. The characteristic colors observed are due to emitted electromagnetic radiation from. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Virtual Flame Test Lab Answer Key Flame Test Lab Answers Quizlet How is it done?, under what. Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? The characteristic colors of light produced when substances are heated. Flame Test Lab Answers Quizlet.
From learningricardo.z19.web.core.windows.net
Flame Test Lab Worksheet Answer Key Chemistry Flame Test Lab Answers Quizlet The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. Flame tests lab flashcards | quizlet. How is it done?, under what. Indicate the specific energy change occurring in the. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test Lab Answers Quizlet Indicate the specific energy change occurring in the ion. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? Why does a metallic ion. Click the card to flip 👆. Study with quizlet and memorize flashcards containing terms like what is a flame test? The lowest?, what are the electrons of. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Flame Test Lab Answer Key Chemistry › Athens Mutual Student Corner Flame Test Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like what is a flame test? How is it done?, under what. Why does a metallic ion. In this lab, you will perform flame tests of several different metal cations. Indicate the specific energy change occurring in the ion. The characteristic colors observed are due to emitted electromagnetic radiation from the excited. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Report Answers Flame Test Lab Answers Quizlet In this lab, you will perform flame tests of several different metal cations. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. The flame tests. Flame Test Lab Answers Quizlet.
From www.studocu.com
Lab report Experiment 1 Flame Test Banawis HJC, Baluyot KJE Flame Test Lab Answers Quizlet How is it done?, under what. Why does a metallic ion. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? The flame tests for sodium sulfate and sodium chloride. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Study with quizlet and memorize flashcards. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Questions Answer Key Flame Test Lab Answers Quizlet The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Flame tests lab flashcards | quizlet. How is it done?, under what. To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: Indicate the specific energy change occurring. Flame Test Lab Answers Quizlet.
From www.studocu.com
Flame Test Lab Report Chemistry for Science Majors Claudia Flame Test Lab Answers Quizlet The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Flame tests lab flashcards | quizlet. In this lab, you will perform flame tests of several different metal cations. To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: Indicate the specific. Flame Test Lab Answers Quizlet.
From slidesharetrick.blogspot.com
Flame Test Lab Answers slidesharetrick Flame Test Lab Answers Quizlet What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? Test the different metal salt solutions in a hot flame and. Study with quizlet and memorize flashcards containing terms like what is a flame test? Why does a metallic ion. How is it done?, under what. The lowest?, what are the. Flame Test Lab Answers Quizlet.
From www.docsity.com
Flame Test Lab Worksheet Activity with Answers Key Exercises Flame Test Lab Answers Quizlet Flame tests lab flashcards | quizlet. In this lab, you will perform flame tests of several different metal cations. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. The flame tests for sodium sulfate and sodium chloride. To help you identify if the cation or anion is responsible for the colors observed during a. Flame Test Lab Answers Quizlet.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test Lab Answers Quizlet Why does a metallic ion. Flame tests lab flashcards | quizlet. Test the different metal salt solutions in a hot flame and. In this lab, you will perform flame tests of several different metal cations. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? The characteristic colors of light produced. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test Lab Answers Quizlet Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? Click the card to flip 👆. To help you identify if the cation or anion is. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Chemistry Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test Lab Answers Quizlet Click the card to flip 👆. In this lab, you will perform flame tests of several different metal cations. Study with quizlet and memorize flashcards containing terms like what is a flame test? The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Why does a metallic ion. How is it done?, under what. Test. Flame Test Lab Answers Quizlet.
From athensmutualaid.net
Chemistry Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test Lab Answers Quizlet Flame tests lab flashcards | quizlet. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Study with quizlet and memorize flashcards containing terms like what is a flame test? Click the card to flip 👆. Test the different metal salt solutions in a hot flame and. Why does a metallic. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Answer Key Flame Test Lab Answers Quizlet To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer the questions below: In this lab, you will perform flame tests of several different metal cations. The flame tests for sodium sulfate and sodium chloride. Indicate the specific energy change occurring in the ion. Test the different metal salt solutions. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Answer Key Flame Test Lab Answers Quizlet The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Click. Flame Test Lab Answers Quizlet.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test Lab Answers Quizlet Why does a metallic ion. Test the different metal salt solutions in a hot flame and. How is it done?, under what. Study with quizlet and memorize flashcards containing terms like what is a flame test? In this lab, you will perform flame tests of several different metal cations. The lowest?, what are the electrons of each elements doing when. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Pre Lab Answer Key Flame Test Lab Answers Quizlet In this lab, you will perform flame tests of several different metal cations. Indicate the specific energy change occurring in the ion. Study with quizlet and memorize flashcards containing terms like what is a flame test? Test the different metal salt solutions in a hot flame and. Since a specific energy is needed to radiate specific light color for every. Flame Test Lab Answers Quizlet.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test Lab Answers Quizlet The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? Why does a metallic ion. Test the different metal salt solutions in a hot flame and. Indicate the specific energy change occurring in the ion. To help you identify if the cation or anion is responsible for the colors observed during. Flame Test Lab Answers Quizlet.
From materialfullpartible.z21.web.core.windows.net
Lab Report Flame Test Flame Test Lab Answers Quizlet The flame tests for sodium sulfate and sodium chloride. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. The lowest?, what are the electrons of each elements doing when you observe the colors of the flame tests? What does a flame test indicate about the energy changes taking place among the electrons in a. Flame Test Lab Answers Quizlet.
From www.studocu.com
Flames+Test+Lab+Worksheet Name Date Flame Test Lab Experimental Flame Test Lab Answers Quizlet The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. Why does a metallic ion. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Study with quizlet and memorize flashcards containing terms like what is a flame test? Click. Flame Test Lab Answers Quizlet.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Answers Quizlet The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. Test. Flame Test Lab Answers Quizlet.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Answers Quizlet Indicate the specific energy change occurring in the ion. The flame tests for sodium sulfate and sodium chloride. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. How is it done?, under what. In this lab, you will perform flame tests of several different. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test For Metals Lab Answer Key Flame Test Lab Answers Quizlet Indicate the specific energy change occurring in the ion. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Click the card to flip 👆. In this lab, you will perform flame tests of several different metal cations. Test the different metal salt solutions in a hot flame and. To help you identify if the. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Chemistry Flame Test Lab Answer Key Pdf Flame Test Lab Answers Quizlet Flame tests lab flashcards | quizlet. The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Click the card to flip 👆. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. The lowest?, what are the electrons of each. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Atomic Emission And Flame Test Lab Answers Flame Test Lab Answers Quizlet Since a specific energy is needed to radiate specific light color for every cation, all compounds containing similar metal cations have the same flame colors. Flame tests lab flashcards | quizlet. The flame tests for sodium sulfate and sodium chloride. To help you identify if the cation or anion is responsible for the colors observed during a flame test, answer. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test Lab Pdf Flame Test Lab Answers Quizlet What does a flame test indicate about the energy changes taking place among the electrons in a metallic ion? Click the card to flip 👆. In this lab, you will perform flame tests of several different metal cations. Study with quizlet and memorize flashcards containing terms like what is a flame test? The characteristic colors observed are due to emitted. Flame Test Lab Answers Quizlet.
From myans.bhantedhammika.net
Flame Test And Atomic Spectra Lab Answer Key Flame Test Lab Answers Quizlet How is it done?, under what. In this lab, you will perform flame tests of several different metal cations. Indicate the specific energy change occurring in the ion. The characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for several. Click the card to flip 👆. The. Flame Test Lab Answers Quizlet.