End Point Error In Titration . There are two types of titration errors, systematic and random errors. Titrations are carried out to. This blog post discusses the most common random and systematic errors that can happen during a. Have you ever wondered why your titration results are not reproducible? Systematic errors can be easily estimated with the help of. First, there is an intrinsic error of the. An uncertainty is the estimated error when taking a measurement. These occur when the measurement scale is not viewed from straight. There are several types of errors that can make titration result differ from the reality. A titration is an experiment that can be used to find the unknown concentration of a solution. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Errors and corrections in titration.
from chem.libretexts.org
Systematic errors can be easily estimated with the help of. Titrations are carried out to. There are two types of titration errors, systematic and random errors. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and systematic errors that can happen during a. There are several types of errors that can make titration result differ from the reality. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. First, there is an intrinsic error of the. Errors and corrections in titration. These occur when the measurement scale is not viewed from straight.
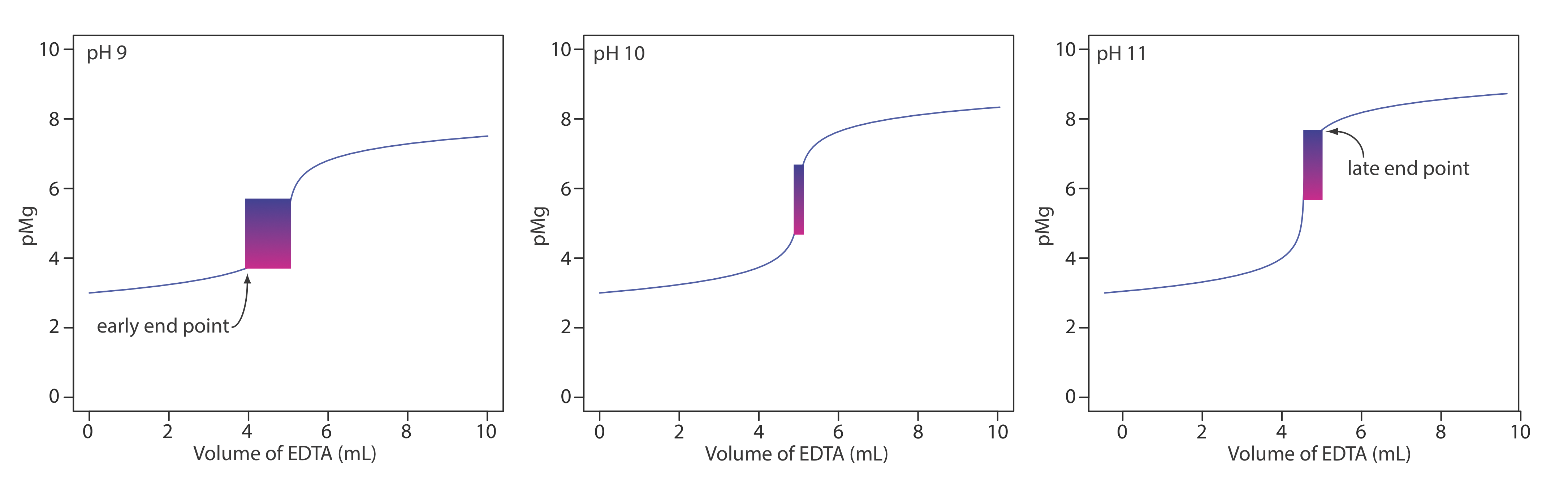
9.3 Complexation Titrations Chemistry LibreTexts
End Point Error In Titration There are two types of titration errors, systematic and random errors. Have you ever wondered why your titration results are not reproducible? There are two types of titration errors, systematic and random errors. Systematic errors can be easily estimated with the help of. There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. Titrations are carried out to. A titration is an experiment that can be used to find the unknown concentration of a solution. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Errors and corrections in titration. These occur when the measurement scale is not viewed from straight. An uncertainty is the estimated error when taking a measurement.
From syatillakmk.blogspot.com
SimplyChemistry Sources of errors in titration End Point Error In Titration There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during a. These occur when the measurement scale is not viewed from straight. A titration is an experiment that can be used to find the unknown concentration of a solution. Errors. End Point Error In Titration.
From www.coursehero.com
[Solved] In a titration, the primary systematic error is the endpoint End Point Error In Titration There are several types of errors that can make titration result differ from the reality. There are two types of titration errors, systematic and random errors. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. First, there is an intrinsic error of the. An uncertainty is the. End Point Error In Titration.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a End Point Error In Titration There are several types of errors that can make titration result differ from the reality. An uncertainty is the estimated error when taking a measurement. Errors and corrections in titration. There are two types of titration errors, systematic and random errors. First, there is an intrinsic error of the. A titration is an experiment that can be used to find. End Point Error In Titration.
From dokumen.tips
(PDF) Computation of intrinsic endpoint errors in titrations with ion End Point Error In Titration There are several types of errors that can make titration result differ from the reality. These occur when the measurement scale is not viewed from straight. This blog post discusses the most common random and systematic errors that can happen during a. First, there is an intrinsic error of the. Systematic errors can be easily estimated with the help of.. End Point Error In Titration.
From www.chegg.com
Solved Fit Page Height 8* Sources of Error in Titrations End Point Error In Titration Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. First, there is an intrinsic error of the. A titration is an experiment that can be used to find the unknown concentration of a solution. There are two types of titration errors, systematic and random errors. There are several. End Point Error In Titration.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts End Point Error In Titration There are several types of errors that can make titration result differ from the reality. Errors and corrections in titration. This blog post discusses the most common random and systematic errors that can happen during a. Titrations are carried out to. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the. End Point Error In Titration.
From www.numerade.com
SOLVED A student is titrating ethanoic acid against sodium hydroxide End Point Error In Titration There are several types of errors that can make titration result differ from the reality. Errors and corrections in titration. Titrations are carried out to. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Have you ever wondered why your titration results are not reproducible? There are two. End Point Error In Titration.
From www.researchgate.net
The relation between alkalinity end point and pH titration curve. End End Point Error In Titration Errors and corrections in titration. These occur when the measurement scale is not viewed from straight. Titrations are carried out to. First, there is an intrinsic error of the. Systematic errors can be easily estimated with the help of. This blog post discusses the most common random and systematic errors that can happen during a. A titration is an experiment. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration Systematic errors can be easily estimated with the help of. There are several types of errors that can make titration result differ from the reality. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and systematic errors that can happen during a. There are two types of titration errors, systematic. End Point Error In Titration.
From www.numerade.com
SOLVED To explain how the result of a titration is affected if you End Point Error In Titration These occur when the measurement scale is not viewed from straight. Titrations are carried out to. Systematic errors can be easily estimated with the help of. A titration is an experiment that can be used to find the unknown concentration of a solution. There are two types of titration errors, systematic and random errors. First, there is an intrinsic error. End Point Error In Titration.
From letitsnowglobe.co.uk
Titration procedure pdf End Point Error In Titration These occur when the measurement scale is not viewed from straight. An uncertainty is the estimated error when taking a measurement. First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. A titration is an experiment that can be used to find the unknown concentration of. End Point Error In Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID2145156 End Point Error In Titration Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Systematic errors can be easily estimated with the help of. First, there is an intrinsic error of the. Have you ever wondered why your titration results are not reproducible? A titration is an experiment that can be used to. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration These occur when the measurement scale is not viewed from straight. Systematic errors can be easily estimated with the help of. An uncertainty is the estimated error when taking a measurement. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. There are several types of errors that can. End Point Error In Titration.
From www.slideserve.com
PPT TITRATION METHODS PowerPoint Presentation, free download ID9641380 End Point Error In Titration Systematic errors can be easily estimated with the help of. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Titrations are carried out to. Have you ever wondered why your titration results are not reproducible? Errors and corrections in titration. There are several types of errors that can. End Point Error In Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point Error In Titration There are two types of titration errors, systematic and random errors. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and systematic errors that can happen during a. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Titrations. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration There are several types of errors that can make titration result differ from the reality. An uncertainty is the estimated error when taking a measurement. Titrations are carried out to. These occur when the measurement scale is not viewed from straight. First, there is an intrinsic error of the. Errors and corrections in titration. Good indicator for a particular reaction. End Point Error In Titration.
From philschatz.com
AcidBase Titrations · Chemistry End Point Error In Titration Titrations are carried out to. Systematic errors can be easily estimated with the help of. Errors and corrections in titration. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. There are several types of errors that can make titration result differ from the reality. An uncertainty is the. End Point Error In Titration.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder End Point Error In Titration A titration is an experiment that can be used to find the unknown concentration of a solution. An uncertainty is the estimated error when taking a measurement. This blog post discusses the most common random and systematic errors that can happen during a. There are several types of errors that can make titration result differ from the reality. There are. End Point Error In Titration.
From www.numerade.com
SOLVED Define the following terms used in volumetric/titrimetric End Point Error In Titration Titrations are carried out to. These occur when the measurement scale is not viewed from straight. An uncertainty is the estimated error when taking a measurement. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. First, there is an intrinsic error of the. This blog post discusses the. End Point Error In Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point Error In Titration First, there is an intrinsic error of the. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. An uncertainty is the estimated error when taking a measurement. These occur when the measurement scale is not viewed from straight. Systematic errors can be easily estimated with the help of.. End Point Error In Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point Error In Titration This blog post discusses the most common random and systematic errors that can happen during a. Have you ever wondered why your titration results are not reproducible? A titration is an experiment that can be used to find the unknown concentration of a solution. Good indicator for a particular reaction is one which gives an end point (colour change) very. End Point Error In Titration.
From slideplayer.com
Chem. 1B 10/6 Lecture. ppt download End Point Error In Titration A titration is an experiment that can be used to find the unknown concentration of a solution. Errors and corrections in titration. Systematic errors can be easily estimated with the help of. An uncertainty is the estimated error when taking a measurement. First, there is an intrinsic error of the. There are two types of titration errors, systematic and random. End Point Error In Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Error In Titration Errors and corrections in titration. Have you ever wondered why your titration results are not reproducible? An uncertainty is the estimated error when taking a measurement. These occur when the measurement scale is not viewed from straight. Titrations are carried out to. Good indicator for a particular reaction is one which gives an end point (colour change) very close to. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration There are two types of titration errors, systematic and random errors. These occur when the measurement scale is not viewed from straight. Have you ever wondered why your titration results are not reproducible? First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. Titrations are carried. End Point Error In Titration.
From www.studocu.com
Mercurimetric titration A systematic error involved, with location of End Point Error In Titration First, there is an intrinsic error of the. There are two types of titration errors, systematic and random errors. These occur when the measurement scale is not viewed from straight. An uncertainty is the estimated error when taking a measurement. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence. End Point Error In Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point Error In Titration Errors and corrections in titration. Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Systematic errors can be easily estimated with the help of. This blog post discusses the most common random and systematic errors that can happen during a. An uncertainty is the estimated error when taking. End Point Error In Titration.
From proper-cooking.info
Endpoint Titration End Point Error In Titration Have you ever wondered why your titration results are not reproducible? Errors and corrections in titration. An uncertainty is the estimated error when taking a measurement. Systematic errors can be easily estimated with the help of. This blog post discusses the most common random and systematic errors that can happen during a. There are two types of titration errors, systematic. End Point Error In Titration.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry End Point Error In Titration First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. An uncertainty is the estimated error when taking a measurement. There are two types of titration errors, systematic and random errors. Good indicator for a particular reaction is one which gives an end point (colour change). End Point Error In Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID End Point Error In Titration Titrations are carried out to. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and systematic errors that can happen during a. These occur when the measurement scale is not viewed from straight. Errors and corrections in titration. There are several types of errors that can make titration result differ. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration First, there is an intrinsic error of the. An uncertainty is the estimated error when taking a measurement. This blog post discusses the most common random and systematic errors that can happen during a. There are several types of errors that can make titration result differ from the reality. There are two types of titration errors, systematic and random errors.. End Point Error In Titration.
From dokumen.tips
(PDF) An Interpretation of the End Point Error in the Potentiometric End Point Error In Titration First, there is an intrinsic error of the. Titrations are carried out to. A titration is an experiment that can be used to find the unknown concentration of a solution. There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during. End Point Error In Titration.
From slideplayer.com
Chapter 7 Let the Titrations Begin ppt download End Point Error In Titration Systematic errors can be easily estimated with the help of. These occur when the measurement scale is not viewed from straight. This blog post discusses the most common random and systematic errors that can happen during a. There are several types of errors that can make titration result differ from the reality. Errors and corrections in titration. There are two. End Point Error In Titration.
From askfilo.com
point, End point, Titration error 2. What is redox titration? Give an exa.. End Point Error In Titration Titrations are carried out to. An uncertainty is the estimated error when taking a measurement. Have you ever wondered why your titration results are not reproducible? There are two types of titration errors, systematic and random errors. This blog post discusses the most common random and systematic errors that can happen during a. Good indicator for a particular reaction is. End Point Error In Titration.
From www.animalia-life.club
Endpoint Titration End Point Error In Titration First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. Have you ever wondered why your titration results are not reproducible? These occur when the measurement scale is not viewed from straight. Good indicator for a particular reaction is one which gives an end point (colour. End Point Error In Titration.
From www.scribd.com
Location of End Point in Potentiometric Argentometric Titration Using End Point Error In Titration Good indicator for a particular reaction is one which gives an end point (colour change) very close to the equivalence point. Titrations are carried out to. A titration is an experiment that can be used to find the unknown concentration of a solution. There are several types of errors that can make titration result differ from the reality. These occur. End Point Error In Titration.