How Many Grams Is Iron Oxide . If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Convert grams fe2o3 to moles. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Visit byju's to understand the properties,.
from www.chegg.com
Visit byju's to understand the properties,. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Convert grams fe2o3 to moles. The molar mass of iron (iii) oxide is 159.69 grams/mole. Mg = 24.3, mgo = 40.3) number of. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. This is found by adding the total mass of iron and oxygen in iron (iii) oxide.
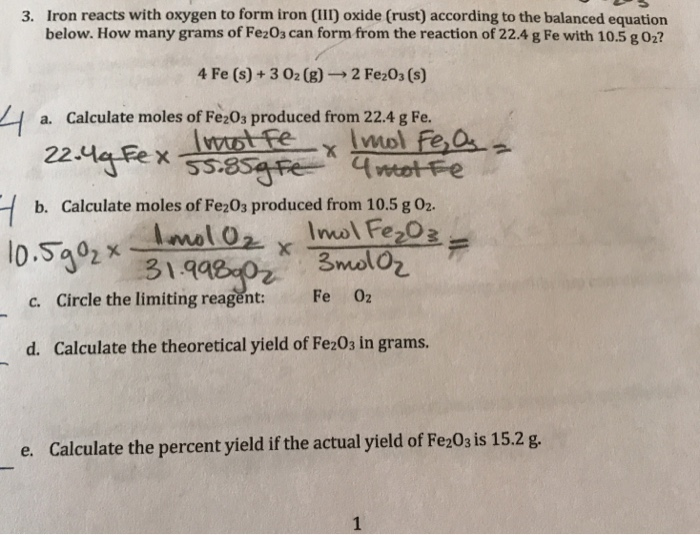
Solved 3. Iron reacts with oxygen to form iron (III) oxide
How Many Grams Is Iron Oxide Convert grams fe2o3 to moles. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Convert grams fe2o3 to moles. Visit byju's to understand the properties,.
From solvedlib.com
The thermite reaction, shown below,is used in welding… SolvedLib How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. The molar mass of iron (iii) oxide is 159.69 grams/mole. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would. How Many Grams Is Iron Oxide.
From wizedu.com
If 87.5g of molten iron (III) oxide reacts with 25.7g of aluminum How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. This. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED According to the following reaction, how many grams of ammonium How Many Grams Is Iron Oxide Visit byju's to understand the properties,. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This is found by adding the total mass of iron and oxygen in. How Many Grams Is Iron Oxide.
From www.numerade.com
A 56g sample of iron reacts with 24 g of oxygen to form how many grams How Many Grams Is Iron Oxide The molar mass of iron (iii) oxide is 159.69 grams/mole. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. This compound is also known as ferric oxide or hematite or iron (iii) oxide.. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED A piece of iron weighing 85.65 g was burned in air. The mass of How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Visit byju's to understand the properties,. Mg = 24.3, mgo = 40.3) number of. This compound is also known as ferric oxide. How Many Grams Is Iron Oxide.
From www.bartleby.com
Answered Consider the balanced chemical reaction… bartleby How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. This compound is also known as ferric oxide or hematite or iron (iii) oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to How Many Grams Is Iron Oxide Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the properties,. Convert grams fe2o3 to moles. The molar mass of iron (iii) oxide is 159.69 grams/mole. This compound is also known as ferric oxide or hematite or iron (iii) oxide. If we were to ask how many grams of elemental iron will be formed by the reduction. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Convert grams fe2o3 to moles. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the. How Many Grams Is Iron Oxide.
From brainly.com
1. How many grams of iron (II) oxide can be produced from 3.4 g of iron How Many Grams Is Iron Oxide Convert grams fe2o3 to moles. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the properties,. Calculate the. How Many Grams Is Iron Oxide.
From www.youtube.com
Mass of iron, Fe, formed from iron oxide, Fe2O3, moles calculation How Many Grams Is Iron Oxide Convert grams fe2o3 to moles. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved How many grams of Fe2+ are present in 3.84 g of How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Visit byju's to understand the properties,. The molar mass of iron (iii) oxide is 159.69 grams/mole. Mg =. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved How many moles of iron are needed to produce 2.59 g How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the properties,. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams fe2o3 to moles. If we were to ask. How Many Grams Is Iron Oxide.
From www.youtube.com
How to Convert Grams Fe to Moles (and Moles Fe to Atoms) YouTube How Many Grams Is Iron Oxide Mg = 24.3, mgo = 40.3) number of. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This compound is also known as ferric oxide or hematite or iron (iii) oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would. How Many Grams Is Iron Oxide.
From www.quora.com
What volume of oxygen gas is required to react completely with 0.539 How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the properties,. Convert grams fe2o3 to moles. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This compound is also known as ferric oxide or hematite or iron (iii). How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED Under certain conditions, the substances iron and oxygen How Many Grams Is Iron Oxide If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Mg = 24.3, mgo = 40.3) number of. Convert. How Many Grams Is Iron Oxide.
From www.slideserve.com
PPT 9. 2 C 3 H 7 OH + 9 O 2 → 6 CO 2 + 8 H 2 O PowerPoint How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. Visit byju's to understand the properties,. Mg = 24.3, mgo = 40.3) number of. Convert grams fe2o3 to moles. The molar mass of iron (iii) oxide is 159.69 grams/mole. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. This is found by adding the total mass of. How Many Grams Is Iron Oxide.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 How Many Grams Is Iron Oxide Convert grams fe2o3 to moles. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Visit byju's to understand the properties,. Mg = 24.3, mgo = 40.3) number of.. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved STOICHIOMETRY PRACTICE SHOW ALL WORK11! 1. Iron How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Visit byju's to understand the properties,. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. This compound is. How Many Grams Is Iron Oxide.
From www.toppr.com
Iron forms two oxides, in first oxide 56 grams of Iron is found to be How Many Grams Is Iron Oxide If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Visit byju's to understand the properties,. Convert grams fe2o3 to moles. The molar mass of iron (iii) oxide is. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED Experiment 3 Report (page 3) Name of the student Student ID How Many Grams Is Iron Oxide If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. The molar mass of iron (iii) oxide is 159.69 grams/mole. Visit byju's to understand the properties,. Mg = 24.3, mgo = 40.3) number of. Convert grams fe2o3 to moles. This is. How Many Grams Is Iron Oxide.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Convert grams fe2o3 to moles. If we were. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED Iron pyrite (FeS2) reacts with oxygen according to the How Many Grams Is Iron Oxide Visit byju's to understand the properties,. Mg = 24.3, mgo = 40.3) number of. The molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams fe2o3 to moles. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. If we were. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a How Many Grams Is Iron Oxide The molar mass of iron (iii) oxide is 159.69 grams/mole. Visit byju's to understand the properties,. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Convert grams fe2o3. How Many Grams Is Iron Oxide.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube How Many Grams Is Iron Oxide The molar mass of iron (iii) oxide is 159.69 grams/mole. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Convert grams fe2o3 to moles. Mg = 24.3, mgo = 40.3) number of. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This compound is also known as ferric oxide or. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVEDThe reaction of iron metal and chlorine gas to give iron(III How Many Grams Is Iron Oxide Mg = 24.3, mgo = 40.3) number of. This compound is also known as ferric oxide or hematite or iron (iii) oxide. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Convert grams fe2o3 to moles. Visit byju's to understand the. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved Consider the balanced chemical reaction below. What How Many Grams Is Iron Oxide If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. This compound is also known as ferric oxide or hematite or iron (iii) oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Calculate the mass of magnesium needed to. How Many Grams Is Iron Oxide.
From camachem.com
What is Iron Oxide and How to Buy Iron Oxide? How Many Grams Is Iron Oxide This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Visit byju's to understand the properties,. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Convert grams fe2o3 to moles. Mg = 24.3, mgo = 40.3) number. How Many Grams Is Iron Oxide.
From www.numerade.com
SOLVEDClassify the type of stoichiometry problem How many grams of How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. Mg = 24.3, mgo = 40.3) number of. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Convert grams fe2o3 to moles. This is found by. How Many Grams Is Iron Oxide.
From www.chegg.com
Solved Under certain conditions, the substances iron and How Many Grams Is Iron Oxide If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams fe2o3 to moles. Visit byju's to understand. How Many Grams Is Iron Oxide.
From www.ceramic-glazes.com
IRON OXIDE Iron (III) Oxide Minium Pigments and Stains How Many Grams Is Iron Oxide Convert grams fe2o3 to moles. This compound is also known as ferric oxide or hematite or iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Visit byju's to understand the properties,. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii). How Many Grams Is Iron Oxide.
From oneclass.com
OneClass How many grams of Iron(III) oxide Fe_2O_3 would contain 25.0 How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. Visit byju's to understand the properties,. Convert grams fe2o3 to. How Many Grams Is Iron Oxide.
From www.indiamart.com
Red Iron Oxide Powder, 100 Gram And 400 Gram, Prasanna Colour Company How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. The molar mass of iron (iii) oxide is 159.69 grams/mole. Mg = 24.3, mgo = 40.3) number of. This compound is also known as ferric oxide or hematite or iron (iii) oxide.. How Many Grams Is Iron Oxide.
From www.slideserve.com
PPT Chapter 6 Chemical Composition Counting Atoms PowerPoint How Many Grams Is Iron Oxide This compound is also known as ferric oxide or hematite or iron (iii) oxide. Visit byju's to understand the properties,. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. The molar mass of. How Many Grams Is Iron Oxide.
From www.numerade.com
A 56g sample of iron reacts with 24 g of oxygen to form how many grams How Many Grams Is Iron Oxide Calculate the mass of magnesium needed to produce 12.1g of magnesium oxide. Mg = 24.3, mgo = 40.3) number of. The molar mass of iron (iii) oxide is 159.69 grams/mole. Visit byju's to understand the properties,. Convert grams fe2o3 to moles. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. If we were. How Many Grams Is Iron Oxide.