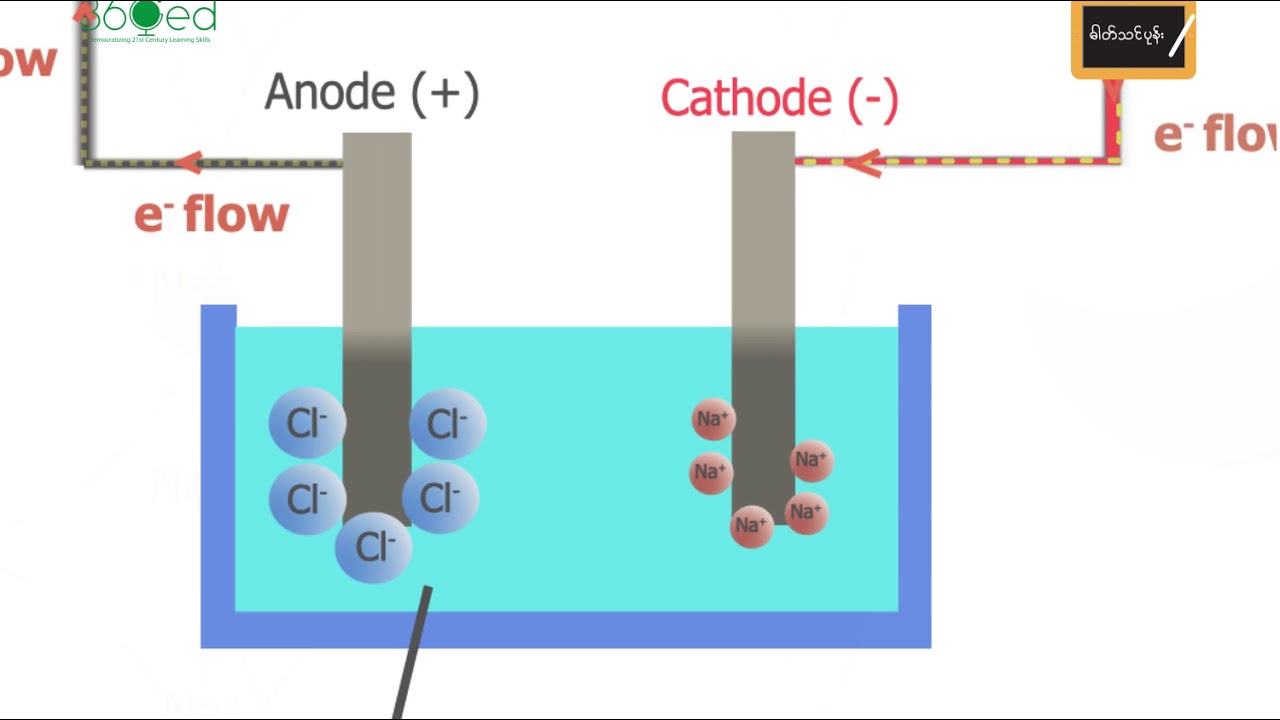
Break Down Nacl Into Electrolyte . electrolysis involves using electricity to break down electrolytes to form elements. This can be seen by comparing nacl with kcl, and nai with ki. a common example of an electrolyte is ordinary salt, sodium chloride. The products of electrolysis can be. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. In each case the compound containing k our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given.
from mungfali.com
In each case the compound containing k when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. a common example of an electrolyte is ordinary salt, sodium chloride. electrolysis involves using electricity to break down electrolytes to form elements. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. The products of electrolysis can be predicted for a given.
Electrolysis Of Sodium Chloride Diagram
Break Down Nacl Into Electrolyte a common example of an electrolyte is ordinary salt, sodium chloride. The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. This can be seen by comparing nacl with kcl, and nai with ki. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. In each case the compound containing k electrolysis involves using electricity to break down electrolytes to form elements. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. a common example of an electrolyte is ordinary salt, sodium chloride.
From www.shutterstock.com
Electrolysis Aqueous Nacl Stock Vector 154945508 Shutterstock Break Down Nacl Into Electrolyte The products of electrolysis can be predicted for a given. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. In each case the compound containing k electrolysis involves using electricity to. Break Down Nacl Into Electrolyte.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Break Down Nacl Into Electrolyte by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. The products of electrolysis can be predicted for a given. electrolysis involves using electricity to break down electrolytes to form elements. our first example of an electrolytic cell will examine how an electric current can be used to break apart. Break Down Nacl Into Electrolyte.
From www.mdpi.com
Energies Free FullText CO2 Fixation by Membrane Separated NaCl Break Down Nacl Into Electrolyte In each case the compound containing k electrolysis involves using electricity to break down electrolytes to form elements. This can be seen by comparing nacl with kcl, and nai with ki. The products of electrolysis can be predicted for a given. a common example of an electrolyte is ordinary salt, sodium chloride. when an electrolyte dissolves, each. Break Down Nacl Into Electrolyte.
From fyozauucn.blob.core.windows.net
What Is Metabolic/Electrolyte Panel at Michael Simmons blog Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. The products of electrolysis can be predicted for a given. electrolysis involves using electricity to break down electrolytes to form elements. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. . Break Down Nacl Into Electrolyte.
From mungfali.com
Sodium Chloride Electrolysis Equation Break Down Nacl Into Electrolyte when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. This can be seen by comparing nacl with kcl, and nai with ki. electrolysis involves. Break Down Nacl Into Electrolyte.
From chem.libretexts.org
17.7 Electrolysis Chemistry LibreTexts Break Down Nacl Into Electrolyte The products of electrolysis can be. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. when an electrolyte dissolves, each type of ion makes an. Break Down Nacl Into Electrolyte.
From www.bartleby.com
Answered The of NaCl(l) into Na(l)… bartleby Break Down Nacl Into Electrolyte in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. In each case the compound containing k electrolysis involves using electricity to break down electrolytes to form elements.. Break Down Nacl Into Electrolyte.
From wisc.pb.unizin.org
D19.3 Solution and Solubility Chemistry 109 Fall 2021 Break Down Nacl Into Electrolyte a common example of an electrolyte is ordinary salt, sodium chloride. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. In each case the compound containing k The products of electrolysis can be. The products of electrolysis can be predicted for a given. when an electrolyte dissolves, each type. Break Down Nacl Into Electrolyte.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Break Down Nacl Into Electrolyte In each case the compound containing k The products of electrolysis can be predicted for a given. a common example of an electrolyte is ordinary salt, sodium chloride. This can be seen by comparing nacl with kcl, and nai with ki. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction. Break Down Nacl Into Electrolyte.
From library.fiveable.me
Electrolysis Intro to Chemistry Class Notes Fiveable Break Down Nacl Into Electrolyte This can be seen by comparing nacl with kcl, and nai with ki. The products of electrolysis can be predicted for a given. The products of electrolysis can be. a common example of an electrolyte is ordinary salt, sodium chloride. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through. Break Down Nacl Into Electrolyte.
From study.com
Electrolysis of Aqueous Solutions Lesson Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. In each case the compound containing k The products of electrolysis can be predicted for a given. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. electrolysis involves using electricity to break down electrolytes to form elements. The. Break Down Nacl Into Electrolyte.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Break Down Nacl Into Electrolyte our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. In each case the compound containing k electrolysis involves using electricity to break down electrolytes to. Break Down Nacl Into Electrolyte.
From chem1180.blogspot.com
CHEM 1180 November 2010 Break Down Nacl Into Electrolyte when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. electrolysis involves using electricity to break down electrolytes to form elements. In each case the compound containing k in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called.. Break Down Nacl Into Electrolyte.
From www.youtube.com
Electrolysis of Sodium Chloride Electrochemistry YouTube Break Down Nacl Into Electrolyte The products of electrolysis can be. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. electrolysis involves using electricity to break. Break Down Nacl Into Electrolyte.
From www.freepik.com
Premium Vector Electrolytes (molten nacl) and electrolytes (nacl Break Down Nacl Into Electrolyte This can be seen by comparing nacl with kcl, and nai with ki. The products of electrolysis can be predicted for a given. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through. Break Down Nacl Into Electrolyte.
From www.youtube.com
Electrolysis of Molten NaCl (in Urdu/Hindi) Oxidation and Reduction Break Down Nacl Into Electrolyte in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. The products of electrolysis can be predicted for a given. This can be seen by comparing nacl with kcl, and nai with ki. a common example of an electrolyte is ordinary salt, sodium chloride. In each case. Break Down Nacl Into Electrolyte.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. This can be seen by comparing nacl with kcl, and nai with ki. our first example of an electrolytic cell will examine how an electric current can be. Break Down Nacl Into Electrolyte.
From www.vrogue.co
Sodium Chloride Electrolysis Equation vrogue.co Break Down Nacl Into Electrolyte The products of electrolysis can be predicted for a given. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. electrolysis involves using electricity to break down electrolytes to form elements. electrolysis involves using electricity to break down electrolytes to form elements. In. Break Down Nacl Into Electrolyte.
From slideplayer.com
Solutions. ppt download Break Down Nacl Into Electrolyte a common example of an electrolyte is ordinary salt, sodium chloride. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. In each case the compound containing k in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called.. Break Down Nacl Into Electrolyte.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Break Down Nacl Into Electrolyte in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. a common example of an electrolyte is ordinary salt, sodium chloride. electrolysis involves using electricity to break down electrolytes to form elements. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its. Break Down Nacl Into Electrolyte.
From slideplayer.com
Chapter 4 Reactions in Aqueous Solution ppt download Break Down Nacl Into Electrolyte our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. This can be seen by comparing nacl with kcl, and nai with ki. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given.. Break Down Nacl Into Electrolyte.
From slideplayer.com
Unit 12 Solutions Section 1 Properties of Solutions ppt download Break Down Nacl Into Electrolyte The products of electrolysis can be predicted for a given. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. In each case the compound containing k The products of electrolysis can be. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements,. Break Down Nacl Into Electrolyte.
From www.slideserve.com
PPT Solution Chemistry The Hydrosphere PowerPoint Presentation, free Break Down Nacl Into Electrolyte The products of electrolysis can be. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. In each case the compound containing k by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. The products of electrolysis. Break Down Nacl Into Electrolyte.
From www.artofit.org
Electrolysis of molten nacl oxidation half and reduction half cell Break Down Nacl Into Electrolyte This can be seen by comparing nacl with kcl, and nai with ki. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. our first example of an electrolytic. Break Down Nacl Into Electrolyte.
From stock.adobe.com
Vector illustration of electrolytic dissociation. Molecules break up Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. a common example of an electrolyte is ordinary salt, sodium chloride. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. The. Break Down Nacl Into Electrolyte.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID3198805 Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. in this case, we have an electrolytic cell where electrical current drives an otherwise nonspontaneous redox reaction through a process called. a common example of an electrolyte is ordinary salt, sodium chloride. by electrolysis, common. Break Down Nacl Into Electrolyte.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Break Down Nacl Into Electrolyte when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. a common example of an electrolyte is ordinary salt, sodium chloride. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. by electrolysis, common. Break Down Nacl Into Electrolyte.
From saylordotorg.github.io
Electrolysis Break Down Nacl Into Electrolyte by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. The products of electrolysis can be. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. a common example of an electrolyte is ordinary salt, sodium. Break Down Nacl Into Electrolyte.
From slideplayer.com
Electrolytes. Ionic compounds Break down into positive and negative Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. This can be seen by comparing nacl with kcl, and nai with ki. by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form. Break Down Nacl Into Electrolyte.
From fyopajikh.blob.core.windows.net
Electrolyte Solutions Examples at Jamaal Perez blog Break Down Nacl Into Electrolyte by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. a common example of an electrolyte is ordinary salt, sodium chloride. The products of electrolysis can be predicted for a given. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. . Break Down Nacl Into Electrolyte.
From www.researchgate.net
Comparison of the electrical conductivity of water and NaCl electrolyte Break Down Nacl Into Electrolyte a common example of an electrolyte is ordinary salt, sodium chloride. The products of electrolysis can be predicted for a given. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. . Break Down Nacl Into Electrolyte.
From slideplayer.com
Electrolytes. Ionic compounds Break down into positive and negative Break Down Nacl Into Electrolyte The products of electrolysis can be. In each case the compound containing k electrolysis involves using electricity to break down electrolytes to form elements. a common example of an electrolyte is ordinary salt, sodium chloride. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound. Break Down Nacl Into Electrolyte.
From www.pinterest.com
Electrolysis of molten NaCl Oxidation half and reduction half cell Break Down Nacl Into Electrolyte electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. when an electrolyte dissolves, each type of ion makes an independent contribution to the current the solution conducts. In each case the compound containing k by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium. Break Down Nacl Into Electrolyte.
From general.chemistrysteps.com
Strong and Weak Electrolytes Chemistry Steps Break Down Nacl Into Electrolyte a common example of an electrolyte is ordinary salt, sodium chloride. In each case the compound containing k The products of electrolysis can be. our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its. when an electrolyte dissolves, each type of ion makes. Break Down Nacl Into Electrolyte.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Break Down Nacl Into Electrolyte The products of electrolysis can be. electrolysis involves using electricity to break down electrolytes to form elements. This can be seen by comparing nacl with kcl, and nai with ki. In each case the compound containing k by electrolysis, common salt, sodium chloride, nacl, can be broken down into its elements, sodium and chlorine. in this case,. Break Down Nacl Into Electrolyte.