Equation In Calorimeter . It's not easy to measure the heat transfer directly. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. It stops once thermal equilibrium between the pan and the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated. heat transfer restores thermal equilibrium once the water and pan are in contact;
from www.youtube.com
heat transfer restores thermal equilibrium once the water and pan are in contact; calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. It's not easy to measure the heat transfer directly. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). It stops once thermal equilibrium between the pan and the.
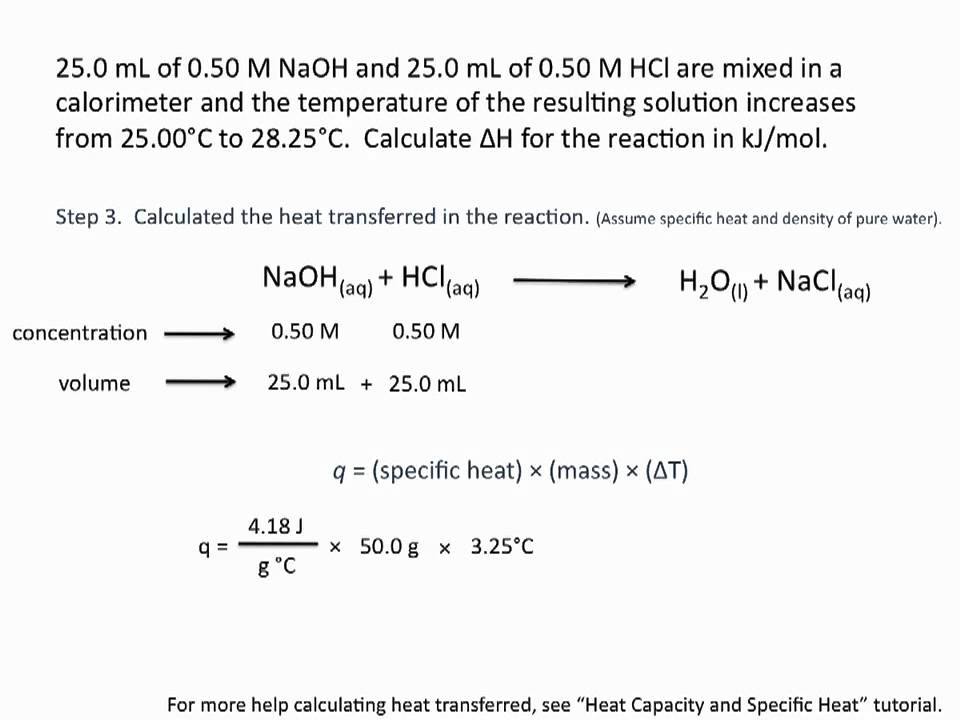
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry
Equation In Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). To do so, the heat is exchanged with a calibrated. calorimetry is used to measure amounts of heat transferred to or from a substance. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. It's not easy to measure the heat transfer directly. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). It stops once thermal equilibrium between the pan and the. heat transfer restores thermal equilibrium once the water and pan are in contact;
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Equation In Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged with a calibrated object (calorimeter). It stops once thermal equilibrium between the pan and the. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q. Equation In Calorimeter.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Equation In Calorimeter use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated. Equation In Calorimeter.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Equation In Calorimeter To do so, the heat is exchanged with a calibrated. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. To do so, the heat is exchanged with a calibrated object (calorimeter). It stops once thermal equilibrium between the pan and the. . Equation In Calorimeter.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Equation In Calorimeter To do so, the heat is exchanged with a calibrated. heat transfer restores thermal equilibrium once the water and pan are in contact; To do so, the heat is exchanged with a calibrated object (calorimeter). calorimetry is used to measure amounts of heat transferred to or from a substance. It stops once thermal equilibrium between the pan and. Equation In Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). It stops once thermal equilibrium between the pan and the. calorimetry is used to measure amounts of heat transferred to or from a substance. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged with. Equation In Calorimeter.
From narodnatribuna.info
Enthalpy Calorimetry Equation Equation In Calorimeter when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). heat transfer restores thermal equilibrium once the water and pan are in contact; To. Equation In Calorimeter.
From lessonlibraryhauliers.z14.web.core.windows.net
How To Solve Calorimetry Problems Chemistry Equation In Calorimeter It stops once thermal equilibrium between the pan and the. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. heat transfer restores thermal equilibrium once the water and pan are in contact; To do so,. Equation In Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Equation In Calorimeter To do so, the heat is exchanged with a calibrated. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial.. Equation In Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum,. Equation In Calorimeter.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Equation In Calorimeter then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged with a calibrated. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the. Equation In Calorimeter.
From frankmccomb.info
Which Statement Describes How a Basic Coffee Cup Calorimeter Works? Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is used to measure amounts of heat transferred to or from a substance.. Equation In Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Equation In Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. It's not easy to measure the heat transfer directly. To do so, the heat is exchanged with a calibrated object (calorimeter). heat transfer restores thermal equilibrium once the water and pan are in contact; when analysing a heat transfer reaction, chemists use the. Equation In Calorimeter.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Equation In Calorimeter To do so, the heat is exchanged with a calibrated. It's not easy to measure the heat transfer directly. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). calorimetry is used to measure amounts of. Equation In Calorimeter.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Equation In Calorimeter It stops once thermal equilibrium between the pan and the. It's not easy to measure the heat transfer directly. heat transfer restores thermal equilibrium once the water and pan are in contact; then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). To do so, the heat is exchanged with a. Equation In Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between the pan and the. calorimetry is used to measure amounts of heat transferred to or from a substance. calculate heat, temperature change, and specific heat after. Equation In Calorimeter.
From exotbfkyp.blob.core.windows.net
What Is Calorimetry And Who Would Be Interested In Performing It at Equation In Calorimeter To do so, the heat is exchanged with a calibrated. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial.. Equation In Calorimeter.
From byjus.com
In a calorimeter (water equivalent=40 g) are 200 g of water and 50 g of Equation In Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). It's not easy to measure the heat transfer directly. when analysing a heat transfer reaction, chemists. Equation In Calorimeter.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Equation In Calorimeter heat transfer restores thermal equilibrium once the water and pan are in contact; calorimetry is used to measure amounts of heat transferred to or from a substance. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). It stops once thermal equilibrium between the pan and the. It's not easy. Equation In Calorimeter.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Equation In Calorimeter then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). heat transfer restores thermal equilibrium once the water and pan are in contact; It's not easy to measure the heat transfer directly. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation. Equation In Calorimeter.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Equation In Calorimeter To do so, the heat is exchanged with a calibrated. It stops once thermal equilibrium between the pan and the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter. Equation In Calorimeter.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Equation In Calorimeter heat transfer restores thermal equilibrium once the water and pan are in contact; when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. It's not easy to measure the heat transfer directly. It stops once thermal equilibrium between the pan and the.. Equation In Calorimeter.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Equation In Calorimeter use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. heat transfer restores thermal equilibrium once the water and pan are in contact; calculate heat, temperature change, and specific heat after thermal equilibrium. Equation In Calorimeter.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. use the equation for heat. Equation In Calorimeter.
From www.youtube.com
Calorimetry calculation YouTube Equation In Calorimeter heat transfer restores thermal equilibrium once the water and pan are in contact; It's not easy to measure the heat transfer directly. To do so, the heat is exchanged with a calibrated object (calorimeter). when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature,. Equation In Calorimeter.
From www.youtube.com
Calorimetry & Enthalpy Problems YouTube Equation In Calorimeter To do so, the heat is exchanged with a calibrated. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. It's not easy to measure the heat transfer directly. It stops once thermal equilibrium between the pan and the. use the equation. Equation In Calorimeter.
From ceglnfrl.blob.core.windows.net
Calorimeter Physics at Daniel Armstrong blog Equation In Calorimeter It's not easy to measure the heat transfer directly. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. To do so, the heat is exchanged with a calibrated. To do so, the heat is exchanged with a calibrated object (calorimeter). then. Equation In Calorimeter.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 Equation In Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. heat transfer restores thermal equilibrium once the water and pan are in contact; calorimetry is used to measure amounts of heat transferred to or from a substance. . Equation In Calorimeter.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Equation In Calorimeter when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). heat transfer restores thermal equilibrium once the water and pan are in contact; To. Equation In Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Equation In Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. It's not easy to measure the heat transfer directly. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. calorimetry is used to measure amounts of heat. Equation In Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Equation In Calorimeter To do so, the heat is exchanged with a calibrated object (calorimeter). calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. It's not easy to measure the heat transfer directly. calorimetry is used to measure amounts of heat transferred to or from a substance. when analysing a heat transfer reaction, chemists use. Equation In Calorimeter.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Equation In Calorimeter heat transfer restores thermal equilibrium once the water and pan are in contact; when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. It stops once thermal equilibrium between the pan and the. then use equation \(ref{5.5.9}\) to determine the heat. Equation In Calorimeter.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Equation In Calorimeter when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. It's not easy to measure the heat transfer directly. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat. Equation In Calorimeter.
From www.youtube.com
How to find calorimeter constant YouTube Equation In Calorimeter It's not easy to measure the heat transfer directly. heat transfer restores thermal equilibrium once the water and pan are in contact; when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. To do so, the heat is exchanged with a calibrated. Equation In Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Equation In Calorimeter It's not easy to measure the heat transfer directly. To do so, the heat is exchanged with a calibrated. To do so, the heat is exchanged with a calibrated object (calorimeter). calorimetry is used to measure amounts of heat transferred to or from a substance. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)). Equation In Calorimeter.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Equation In Calorimeter To do so, the heat is exchanged with a calibrated. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific. use the equation for heat transfer \(q. Equation In Calorimeter.