Evaluation For Calorimetry Experiment . Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Measure the amount of heat involved in frostbite. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Determination of calorimeter constant 1. As part of this lab, you will: This deals with topic 5.1, a calorimetry. This months post will take up where the others have left off and look at the next skill. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry is used to measure amounts of heat. Obtain or assemble a calorimeter as shown in figure 9.
from www.studocu.com
Determination of calorimeter constant 1. Obtain or assemble a calorimeter as shown in figure 9. This months post will take up where the others have left off and look at the next skill. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Measure the amount of heat involved in frostbite. As part of this lab, you will: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. This deals with topic 5.1, a calorimetry.
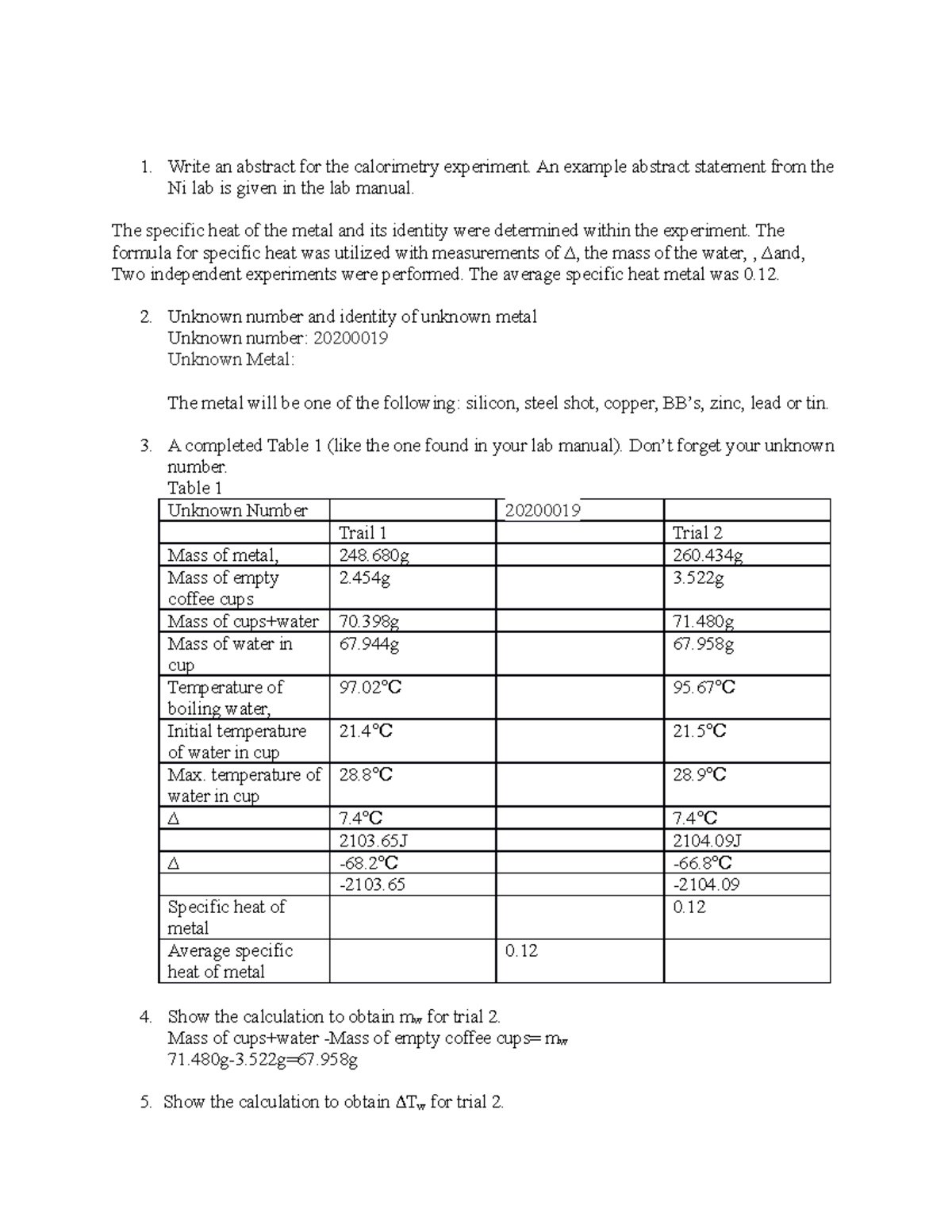
Calorimetry Lab Report Write an abstract for the calorimetry
Evaluation For Calorimetry Experiment One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This deals with topic 5.1, a calorimetry. Obtain or assemble a calorimeter as shown in figure 9. Measure the amount of heat involved in frostbite. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. This months post will take up where the others have left off and look at the next skill. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Determination of calorimeter constant 1. Calorimetry is used to measure amounts of heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Evaluation For Calorimetry Experiment Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Obtain or assemble a calorimeter as shown in figure 9. In this experiment you will heat. Evaluation For Calorimetry Experiment.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Evaluation For Calorimetry Experiment This months post will take up where the others have left off and look at the next skill. As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Evaluation For Calorimetry Experiment.
From studylib.net
Calorimetry Lab Specific Heat Capacity Evaluation For Calorimetry Experiment Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetry is used to measure amounts of heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water. Evaluation For Calorimetry Experiment.
From studylib.net
Calorimetry Project. Project Guidelines Evaluation For Calorimetry Experiment As part of this lab, you will: This months post will take up where the others have left off and look at the next skill. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Evaluation For Calorimetry Experiment.
From www.youtube.com
Summative Assessment Calorimetry Simulation YouTube Evaluation For Calorimetry Experiment Obtain or assemble a calorimeter as shown in figure 9. This months post will take up where the others have left off and look at the next skill. Determination of calorimeter constant 1. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. One technique we. Evaluation For Calorimetry Experiment.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Evaluation For Calorimetry Experiment Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. As part of this lab, you will: This months post will take up where the others have left off and. Evaluation For Calorimetry Experiment.
From www.pinterest.com
Calorimetry CER Assessment 4 Versions w/ Key and Rubric Chemistry Evaluation For Calorimetry Experiment One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Determination of calorimeter constant 1. Obtain or assemble a calorimeter as shown in figure. Evaluation For Calorimetry Experiment.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Evaluation For Calorimetry Experiment Calorimetry is used to measure amounts of heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this. Evaluation For Calorimetry Experiment.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Evaluation For Calorimetry Experiment One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. This months post will take up where the others. Evaluation For Calorimetry Experiment.
From studylib.net
Lab Session 9, Experiment 8 Calorimetry, Heat of Reaction Evaluation For Calorimetry Experiment Determination of calorimeter constant 1. Calorimetry is used to measure amounts of heat. This months post will take up where the others have left off and look at the next skill. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. One technique we can use. Evaluation For Calorimetry Experiment.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Evaluation For Calorimetry Experiment This months post will take up where the others have left off and look at the next skill. Calorimetry is used to measure amounts of heat. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. As part of this lab, you will: Measure the amount of heat. Evaluation For Calorimetry Experiment.
From users.highland.edu
Calorimetry Evaluation For Calorimetry Experiment In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Determination of calorimeter constant 1. This months post will take up where the others have left off and look at the next skill. Calorimetry is the study of heat transferred in a chemical reaction, and a. Evaluation For Calorimetry Experiment.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Dato lob Sec Evaluation For Calorimetry Experiment As part of this lab, you will: Obtain or assemble a calorimeter as shown in figure 9. This months post will take up where the others have left off and look at the next skill. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry. Evaluation For Calorimetry Experiment.
From studylib.net
Calorimetry experiment 1 Evaluation For Calorimetry Experiment Obtain or assemble a calorimeter as shown in figure 9. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This months post will. Evaluation For Calorimetry Experiment.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Evaluation For Calorimetry Experiment Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Obtain or assemble a calorimeter as shown in figure 9. Calorimetry is used to. Evaluation For Calorimetry Experiment.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Evaluation For Calorimetry Experiment As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Determination of calorimeter constant 1. This months post will take up where the others have left off and look at the next skill. This deals with topic 5.1, a calorimetry. Measure. Evaluation For Calorimetry Experiment.
From www.scribd.com
Calorimetry Experiment PDF Heat Enthalpy Evaluation For Calorimetry Experiment Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Evaluation For Calorimetry Experiment.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Evaluation For Calorimetry Experiment Obtain or assemble a calorimeter as shown in figure 9. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Measure the amount of heat involved in frostbite. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. This deals with topic. Evaluation For Calorimetry Experiment.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Evaluation For Calorimetry Experiment As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. This months post will take up where the others have left off and look at the next skill. Calorimetry is used to measure amounts of heat. Obtain or assemble a calorimeter. Evaluation For Calorimetry Experiment.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic Evaluation For Calorimetry Experiment One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Measure the amount of heat involved. Evaluation For Calorimetry Experiment.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Evaluation For Calorimetry Experiment This months post will take up where the others have left off and look at the next skill. Measure the amount of heat involved in frostbite. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Determination of calorimeter constant 1. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot. Evaluation For Calorimetry Experiment.
From studylib.net
experiment 9 Thermochemistry Calorimetry Evaluation For Calorimetry Experiment This deals with topic 5.1, a calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Obtain or assemble a calorimeter as shown in figure 9. Determination of calorimeter constant 1. Measure the amount of heat involved in frostbite.. Evaluation For Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Evaluation For Calorimetry Experiment Measure the amount of heat involved in frostbite. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry is used to measure amounts of heat. Determination of calorimeter constant 1. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using. Evaluation For Calorimetry Experiment.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Evaluation For Calorimetry Experiment Calorimetry is used to measure amounts of heat. As part of this lab, you will: Determination of calorimeter constant 1. This deals with topic 5.1, a calorimetry. Obtain or assemble a calorimeter as shown in figure 9. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. Evaluation For Calorimetry Experiment.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Evaluation For Calorimetry Experiment Determination of calorimeter constant 1. Obtain or assemble a calorimeter as shown in figure 9. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This deals with topic 5.1, a calorimetry. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured. Evaluation For Calorimetry Experiment.
From www.researchgate.net
Differential scanning calorimetry (DSC) heat flow plots for individual Evaluation For Calorimetry Experiment This deals with topic 5.1, a calorimetry. Calorimetry is used to measure amounts of heat. Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then. Evaluation For Calorimetry Experiment.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Evaluation For Calorimetry Experiment Determination of calorimeter constant 1. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry is used to measure amounts of heat. Obtain. Evaluation For Calorimetry Experiment.
From www.studocu.com
Experiment 25 calorimetry Bryce Augustine Chem 005 Experiment 25 Evaluation For Calorimetry Experiment In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. As part of this lab, you will: This deals with topic 5.1, a calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. One technique we can use to. Evaluation For Calorimetry Experiment.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Evaluation For Calorimetry Experiment Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry is used to measure amounts of heat. This months post will take up where the others have left off and look at the next skill. As part of this lab, you will: In this experiment. Evaluation For Calorimetry Experiment.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Evaluation For Calorimetry Experiment Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Measure the amount of heat involved in frostbite. This deals with topic 5.1, a calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. One technique we can use to measure. Evaluation For Calorimetry Experiment.
From studylib.net
Lab 33 calorimetry lab Evaluation For Calorimetry Experiment One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of calorimeter constant 1. This deals with topic 5.1, a calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetry is used to measure amounts of heat. Calorimetry is. Evaluation For Calorimetry Experiment.
From www.nagwa.com
Question Video Determining the Suitability of Experimental Apparatus Evaluation For Calorimetry Experiment Determination of calorimeter constant 1. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. As part of this lab, you will: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a.. Evaluation For Calorimetry Experiment.
From www.scribd.com
Analyzing Calorimetry Experiments An Assessment of Chemical Reactions Evaluation For Calorimetry Experiment Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐3 consider what happens when. Evaluation For Calorimetry Experiment.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Evaluation For Calorimetry Experiment Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This months post will take up where the others have left off and look. Evaluation For Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Evaluation For Calorimetry Experiment This months post will take up where the others have left off and look at the next skill. Calorimetry is used to measure amounts of heat. As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Determination of calorimeter constant 1.. Evaluation For Calorimetry Experiment.