Iron (Iii) Chloride Dihydrate Formula . In the solid, these water. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Iron (iii) chloride is typically produced on an industrial scale. The equation for this reaction is: An older system of nomenclature for such cations is still widely used, however. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Some of the metals form very common ions which have latin names that are in common use, and you. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). So fe +2 is iron (ii) and fe +3 is iron (iii).
from www.chegg.com
If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such cations is still widely used, however. The equation for this reaction is: Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. So fe +2 is iron (ii) and fe +3 is iron (iii). Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. In the solid, these water. Iron (iii) chloride is typically produced on an industrial scale. Some of the metals form very common ions which have latin names that are in common use, and you.
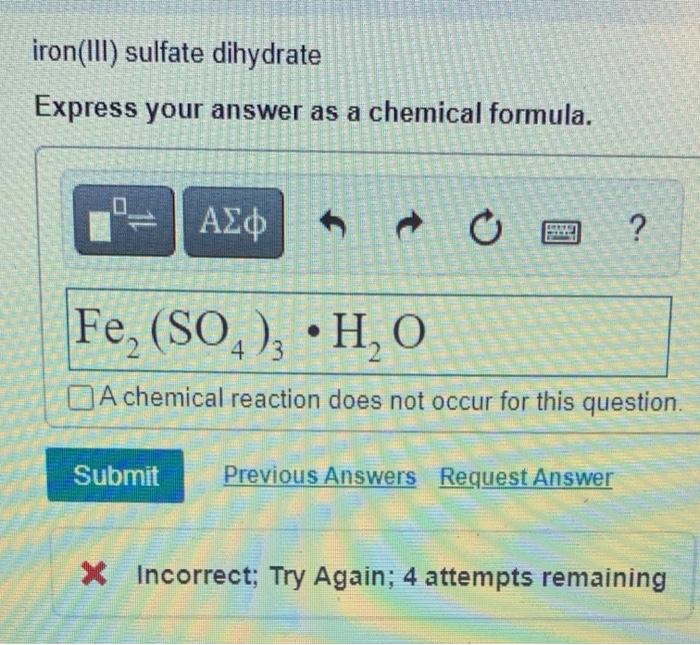
Solved iron(III) sulfate dihydrate Express your answer as a
Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. So fe +2 is iron (ii) and fe +3 is iron (iii). If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such cations is still widely used, however. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Some of the metals form very common ions which have latin names that are in common use, and you. Iron (iii) chloride is typically produced on an industrial scale. In the solid, these water. The equation for this reaction is: Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas.
From app.pandai.org
Chemical Formulae Iron (Iii) Chloride Dihydrate Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Some of the metals form very common ions which have latin names that are in common use, and you. So fe +2 is iron (ii) and fe +3 is iron (iii). An older system. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
Iron(III) chloride YouTube Iron (Iii) Chloride Dihydrate Formula Iron (iii) chloride is typically produced on an industrial scale. So fe +2 is iron (ii) and fe +3 is iron (iii). If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Some of the metals form very common ions which have latin names that are in common use, and you. Hydrated ionic compounds (i.e., hydrates). Iron (Iii) Chloride Dihydrate Formula.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Iron (Iii) Chloride Dihydrate Formula In the solid, these water. Iron (iii) chloride is typically produced on an industrial scale. Some of the metals form very common ions which have latin names that are in common use, and you. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. So fe +2 is iron (ii) and fe +3 is iron (iii). An older. Iron (Iii) Chloride Dihydrate Formula.
From slideplayer.com
Chapter 7 Compounds and Their Bonds ppt download Iron (Iii) Chloride Dihydrate Formula Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Iron (iii) chloride is typically produced on an industrial scale. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. Iron (Iii) Chloride Dihydrate Formula.
From www.fishersci.se
Iron(III) chloride, 98, pure, anhydrous, Thermo Scientific Chemicals Iron (Iii) Chloride Dihydrate Formula In the solid, these water. Some of the metals form very common ions which have latin names that are in common use, and you. Iron (iii) chloride is typically produced on an industrial scale. So fe +2 is iron (ii) and fe +3 is iron (iii). If the metal can form ions with different charges, a roman numeral in parentheses. Iron (Iii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED Watch the pictures of the following ionic compounds in the Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. So fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in common use, and you. Iron (iii) chloride is typically produced on an industrial scale. If the metal can form ions with. Iron (Iii) Chloride Dihydrate Formula.
From www.sciencephoto.com
Anhydrous iron (III) chloride Stock Image C009/9429 Science Photo Iron (Iii) Chloride Dihydrate Formula If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. In the solid, these water. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) chloride is typically produced on. Iron (Iii) Chloride Dihydrate Formula.
From www.fishersci.co.uk
Iron(III) chloride hexahydrate, 99+, for analysis, Thermo Scientific Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Iron (iii) chloride is typically produced on an industrial scale. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. So fe +2. Iron (Iii) Chloride Dihydrate Formula.
From www.scientificlabs.co.uk
Iron(III) chloride, reagent gr 1577405G SIGMAALDRICH SLS Iron (Iii) Chloride Dihydrate Formula In the solid, these water. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. An older system. Iron (Iii) Chloride Dihydrate Formula.
From www.nanochemazone.com
Iron III Chloride Hexahydrate Nanochemazone 10025771 Iron (Iii) Chloride Dihydrate Formula In the solid, these water. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+. Iron (Iii) Chloride Dihydrate Formula.
From molekula.com
Purchase Iron(III) chloride anhydrous [7705080] online • Catalog Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Hydrated ionic compounds (i.e., hydrates). Iron (Iii) Chloride Dihydrate Formula.
From www.merck-lifescience.com.vn
IRON(III) CHLORIDE, REAGE NT GRADE, 97 Merck Life Science Vietnam Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Iron (iii) chloride is typically produced on an industrial scale. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Some. Iron (Iii) Chloride Dihydrate Formula.
From www.chegg.com
Solved 2. Iron (III) chloride dissolves in water forming Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. An older system of nomenclature for such cations is still widely used, however. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas.. Iron (Iii) Chloride Dihydrate Formula.
From www.libertysci.com
Iron(III) Chloride, hexahydrate, 25 g Liberty Scientific Iron (Iii) Chloride Dihydrate Formula So fe +2 is iron (ii) and fe +3 is iron (iii). In the solid, these water. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Iron (iii) chloride is typically produced on an industrial scale. Some of the metals form very common ions which. Iron (Iii) Chloride Dihydrate Formula.
From fphoto.photoshelter.com
science chemistry compound iron iii chloride Fundamental Photographs Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. So fe +2 is iron (ii) and fe +3 is iron (iii). Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is. Iron (Iii) Chloride Dihydrate Formula.
From www.sigmaaldrich.id
IRON(III) CHLORIDE HEXAHYDRATE, ACS REA& Merck Life Science Indonesia Iron (Iii) Chloride Dihydrate Formula Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Iron (iii) chloride is typically produced on an industrial scale. Some of the metals form very common ions which have latin names that are in common use, and you. So fe +2 is iron (ii) and fe +3 is iron (iii). If the metal. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
Lewis Structure of Iron (III) Chloride, FeCl3 YouTube Iron (Iii) Chloride Dihydrate Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). In the solid, these water. So fe +2 is iron (ii) and fe +3 is iron (iii). Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Some of the metals form. Iron (Iii) Chloride Dihydrate Formula.
From lab.honeywell.com
Iron(III) chloride hexahydrate 31232 Honeywell Research Chemicals Iron (Iii) Chloride Dihydrate Formula If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Iron (iii) chloride is typically produced on an industrial scale. The equation for this reaction is: Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Thus cu + is. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
How to write the formula for iron (III) chloride YouTube Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. The equation for this reaction is: Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. In the solid, these water. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
Write the formula for iron(III) chloride YouTube Iron (Iii) Chloride Dihydrate Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Iron (iii) chloride is typically produced on an industrial scale. An older system of nomenclature for such cations is still widely used, however. So fe +2 is iron (ii) and fe +3 is iron. Iron (Iii) Chloride Dihydrate Formula.
From www.chegg.com
Solved iron(III) sulfate dihydrate Express your answer as a Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. Some of the metals form very common ions which have latin names that are in common use, and you. Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Iron (iii) chloride is typically produced on an industrial scale. Thus cu + is copper(i) (read. Iron (Iii) Chloride Dihydrate Formula.
From 1malaysiabiolab.com
Iron (III) Chloride Hexahydrate 1Malaysia Bio Lab Iron (Iii) Chloride Dihydrate Formula The equation for this reaction is: Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) chloride is typically produced on an industrial scale. In the solid, these water. Fecl. Iron (Iii) Chloride Dihydrate Formula.
From molekula.com
Purchase Iron(III) chloride hexahydrate [10025771] online • Catalog Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. In the solid, these water. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is. Iron (Iii) Chloride Dihydrate Formula.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron (Iii) Chloride Dihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you. In the solid, these water. So fe +2 is iron (ii) and fe +3 is iron (iii). Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Iron (iii) chloride is typically produced on an. Iron (Iii) Chloride Dihydrate Formula.
From www.chegg.com
Solved When iron (III) chloride and hydrogen sulfide react Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. In the solid, these water. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. The equation for this reaction is: If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to. Iron (Iii) Chloride Dihydrate Formula.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron (Iii) Chloride Dihydrate Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Some of the metals form very common ions which have latin names that are in common use, and you. In the solid, these water. Fecl 3 + 3h 2 o → fe (oh) 3. Iron (Iii) Chloride Dihydrate Formula.
From www.shutterstock.com
Iron Iii Chloride Handwritten Chemical Formula Stock Illustration Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. An older system of nomenclature for such cations is still widely used, however. So fe +2 is iron (ii) and fe +3 is iron (iii). The equation for this reaction is: Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. If. Iron (Iii) Chloride Dihydrate Formula.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download Iron (Iii) Chloride Dihydrate Formula In the solid, these water. The equation for this reaction is: Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. So fe +2 is iron (ii) and fe +3 is iron (iii). Thus cu + is copper(i) (read as “copper one”),. Iron (Iii) Chloride Dihydrate Formula.
From www.toppr.com
Iron III Chloride Formula Structure, Preparations and Properties Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. So fe +2 is iron (ii) and fe +3 is iron (iii). Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Some of the metals form very common ions which have latin names that are in common use, and you. In the solid, these. Iron (Iii) Chloride Dihydrate Formula.
From www.chegg.com
Solved A 5.49 g sample of an iron (III) chloride hydrate was Iron (Iii) Chloride Dihydrate Formula Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Hydrated ionic compounds (i.e., hydrates) have a specfic number of water molecules in their chemical formulas. Iron (iii) chloride is typically produced. Iron (Iii) Chloride Dihydrate Formula.
From hamptonresearch.com
Hampton Research Iron (Iii) Chloride Dihydrate Formula If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Some of the metals form very common ions which have latin names that are in common use, and you. Iron (iii) chloride is typically produced on an industrial scale. In the solid, these water. Hydrated ionic. Iron (Iii) Chloride Dihydrate Formula.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Iron (Iii) Chloride Dihydrate Formula Fecl 3 + 3h 2 o → fe (oh) 3 + 3hcl. Some of the metals form very common ions which have latin names that are in common use, and you. The equation for this reaction is: Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn. Iron (Iii) Chloride Dihydrate Formula.
From www.transtutors.com
(Get Answer) The Name Of FeCl3 X 6 H2O Is? Iron Chloride Iron (III Iron (Iii) Chloride Dihydrate Formula An older system of nomenclature for such cations is still widely used, however. Iron (iii) chloride is typically produced on an industrial scale. Some of the metals form very common ions which have latin names that are in common use, and you. Thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn. Iron (Iii) Chloride Dihydrate Formula.