Tlc Solvent Polarity . The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. If your compounds stay in the baseline while using typical solvent. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : High polarity solvents will do. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. Low polarity solvents will cause the spots to move more slowly. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Explore tlc with an interactive simulator: How do you tlc extremely polar compounds? As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity.
from www.bartleby.com
A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. Explore tlc with an interactive simulator: How do you tlc extremely polar compounds? Low polarity solvents will cause the spots to move more slowly. If your compounds stay in the baseline while using typical solvent. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). High polarity solvents will do. In all forms of chromatography, samples equilibrate between stationary and mobile phases.
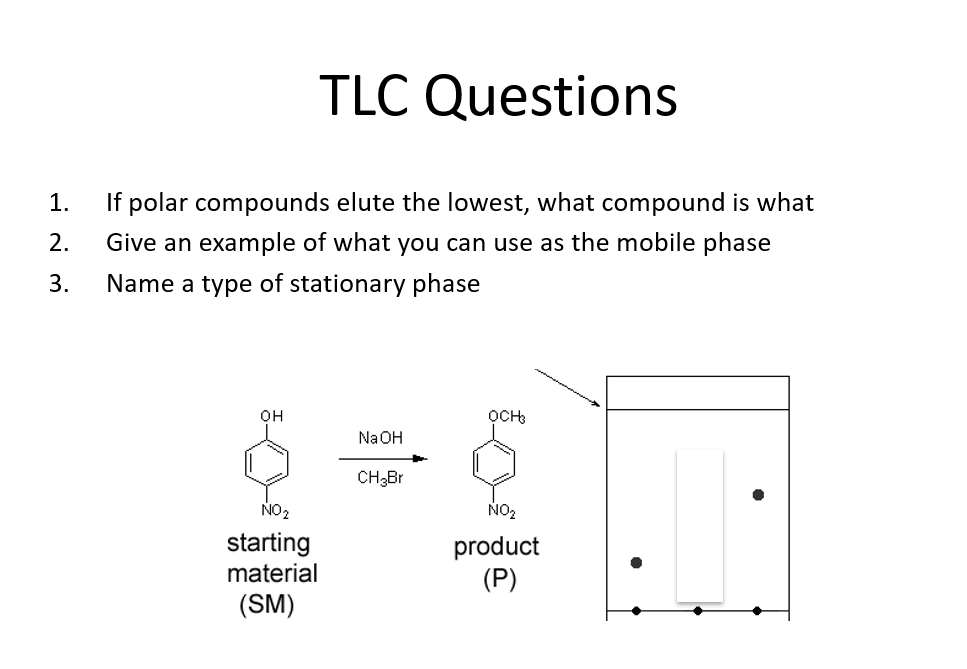
Answered TLC Questions 1. If polar compounds… bartleby
Tlc Solvent Polarity High polarity solvents will do. Explore tlc with an interactive simulator: As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. Low polarity solvents will cause the spots to move more slowly. How do you tlc extremely polar compounds? High polarity solvents will do. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : If your compounds stay in the baseline while using typical solvent. In all forms of chromatography, samples equilibrate between stationary and mobile phases.
From drive.google.com
TLC Solvents polarity.pdf Google Drive Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. How do you tlc extremely polar compounds? To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The eluting strength. Tlc Solvent Polarity.
From mungfali.com
Thin Layer Chromatography Polarity Tlc Solvent Polarity The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. In all forms of chromatography, samples equilibrate between stationary and mobile phases. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. High polarity solvents will do. A solvent that can be used for. Tlc Solvent Polarity.
From www.numerade.com
SOLVED What does the above TLC experiment tell you about the effect of Tlc Solvent Polarity The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : How do you tlc extremely polar compounds? In all forms of chromatography, samples equilibrate between stationary and mobile phases. To separate. Tlc Solvent Polarity.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Tlc Solvent Polarity To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. The eluting strength of. Tlc Solvent Polarity.
From www.bartleby.com
Answered TLC Questions 1. If polar compounds… bartleby Tlc Solvent Polarity In all forms of chromatography, samples equilibrate between stationary and mobile phases. How do you tlc extremely polar compounds? In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). The eluting strength of a. Tlc Solvent Polarity.
From www.reddit.com
TLC and solvent polarity!! What does it mean that it’s ‘too non polar Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. If your compounds stay in the baseline while using typical solvent. In all forms of chromatography, samples equilibrate between stationary and mobile phases. A solvent that can be used for separating mixtures of strongly polar. Tlc Solvent Polarity.
From www.chegg.com
Solved Why is it that changing the solvent in normal phase Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. High polarity solvents will do. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : In almost all applications of. Tlc Solvent Polarity.
From mavink.com
Solvent Polarity Chart For Tlc Tlc Solvent Polarity High polarity solvents will do. How do you tlc extremely polar compounds? In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). To separate strongly basic components, make a mixture of 10% nh 4. Tlc Solvent Polarity.
From www.chegg.com
Solved D Question 5 5 pts The TLC plate is more polar than Tlc Solvent Polarity A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. How do you tlc extremely polar compounds?. Tlc Solvent Polarity.
From www.researchgate.net
Representative thinlayer chromatography (TLC) plate of buttermilk Tlc Solvent Polarity To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. As with crystallization, solvent mixtures can be used to achieve intermediate. Tlc Solvent Polarity.
From www.researchgate.net
Nature of solvents, its polarity and general uses Download Scientific Tlc Solvent Polarity In all forms of chromatography, samples equilibrate between stationary and mobile phases. The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. High polarity solvents will do. Explore tlc with an interactive simulator: A solvent that can be used for separating. Tlc Solvent Polarity.
From alexmistry.z13.web.core.windows.net
Order Of Solvent Polarity Tlc Solvent Polarity The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. High polarity solvents will do. Low polarity solvents will cause the spots to move more slowly. How do you tlc extremely polar compounds? In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent. Tlc Solvent Polarity.
From www.numerade.com
Solvent Solvent Polarity Index, P Hexane 0.1 Carbon tetrachloride Tlc Solvent Polarity How do you tlc extremely polar compounds? Explore tlc with an interactive simulator: If your compounds stay in the baseline while using typical solvent. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. In almost all applications of tlc, the stationary phase. Tlc Solvent Polarity.
From gbu-presnenskij.ru
TLC And Solvent Polarity!! What Does It Mean That It's 'too, 42 OFF Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : How do you tlc extremely polar compounds? In all forms of chromatography, samples equilibrate between stationary and mobile phases. To separate strongly basic components, make a mixture of 10% nh 4 oh. Tlc Solvent Polarity.
From microbenotes.com
Thin Layer Chromatography Principle, Parts, Steps, Uses Tlc Solvent Polarity If your compounds stay in the baseline while using typical solvent. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. How do you tlc extremely polar compounds? Low. Tlc Solvent Polarity.
From mavink.com
Solvent Polarity Chart For Tlc Tlc Solvent Polarity As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. How do you tlc extremely polar compounds? In all forms of chromatography, samples equilibrate between stationary and mobile phases. Low polarity solvents will cause the spots to move more slowly. Explore tlc with an interactive simulator: The eluting strength of a solvent is primarily related to. Tlc Solvent Polarity.
From gbu-presnenskij.ru
TLC And Solvent Polarity!! What Does It Mean That It's 'too, 42 OFF Tlc Solvent Polarity In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). Low polarity solvents will cause the spots to move more slowly. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of. Tlc Solvent Polarity.
From mavink.com
Polarity Chart Of Solvents Tlc Solvent Polarity The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. Low polarity solvents will cause the spots to move more slowly. High polarity solvents will do. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. How. Tlc Solvent Polarity.
From www.brainkart.com
Choice Of Solvent System in Thin Layer Chromatography (TLC) Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Explore tlc with an interactive simulator: If your compounds stay in the baseline while using typical solvent. High polarity solvents will do. In. Tlc Solvent Polarity.
From www.slideserve.com
PPT Organic I Lab PowerPoint Presentation, free download ID2150796 Tlc Solvent Polarity Explore tlc with an interactive simulator: A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : If your compounds stay in the baseline while using typical solvent. Low polarity solvents will cause the spots to move more slowly. In all forms of chromatography, samples equilibrate between stationary and mobile phases. In almost all. Tlc Solvent Polarity.
From www.researchgate.net
TLC tracing showing compounds in various solvent systems. TLC thin Tlc Solvent Polarity As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. How do you tlc extremely polar compounds? To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. Low polarity solvents will cause the spots to move more slowly. High. Tlc Solvent Polarity.
From www.makingmolecules.com
Thin Layer Chromatography (TLC) — Making Molecules Tlc Solvent Polarity The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : If your compounds stay in the baseline while using typical solvent. How do you. Tlc Solvent Polarity.
From www.slideshare.net
Chromatography Tlc Solvent Polarity If your compounds stay in the baseline while using typical solvent. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. High polarity solvents will do. The solvent for a chromatographic separation will usually be specified in the script however a good starting. Tlc Solvent Polarity.
From www.chegg.com
Solved 2. You ran a TLC of a mixture of three compounds in Tlc Solvent Polarity A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. To separate strongly basic components, make a mixture of 10% nh 4 oh in. Tlc Solvent Polarity.
From www.researchgate.net
TLC profile of T. involucrata L. Eluting solvents Fractions TLC solvent Tlc Solvent Polarity Low polarity solvents will cause the spots to move more slowly. Explore tlc with an interactive simulator: How do you tlc extremely polar compounds? To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. High polarity solvents will do. The solvent for a. Tlc Solvent Polarity.
From owlcation.com
Thin Layer Chromatography (TLC) Principle and Procedure Owlcation Tlc Solvent Polarity As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. How do you tlc extremely polar compounds? If your compounds stay in the baseline while using typical solvent. High polarity solvents will do. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture. Tlc Solvent Polarity.
From ponasa.condesan-ecoandes.org
Solvent Polarity Chart Pdf Ponasa Tlc Solvent Polarity In all forms of chromatography, samples equilibrate between stationary and mobile phases. As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. The solvent for a chromatographic separation will usually be specified in the script however a good starting point for a new separation is one with medium polarity such as. High polarity solvents will do.. Tlc Solvent Polarity.
From alexmistry.z13.web.core.windows.net
Polarity Of Solvents In Chromatography Tlc Solvent Polarity To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in dichlormethane. High polarity solvents will do. If your compounds stay in the baseline while using typical solvent. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. Tlc Solvent Polarity.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Tlc Solvent Polarity How do you tlc extremely polar compounds? The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. High polarity solvents will do. To separate strongly basic components, make a mixture of 10% nh 4 oh in methanol, and then make a 1 to 10% mixture of this in. Tlc Solvent Polarity.
From www.numerade.com
SOLVED How does the polarity of the solvent affect TLC and Column Tlc Solvent Polarity How do you tlc extremely polar compounds? In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate :. Tlc Solvent Polarity.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Tlc Solvent Polarity The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). A solvent. Tlc Solvent Polarity.
From www.slideserve.com
PPT TLC and Dihydroxylation PowerPoint Presentation, free download Tlc Solvent Polarity As with crystallization, solvent mixtures can be used to achieve intermediate degrees of polarity. High polarity solvents will do. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). Explore tlc with an interactive. Tlc Solvent Polarity.
From stock-picture.com
thin layer chromatography image Big Image Stock Tlc Solvent Polarity If your compounds stay in the baseline while using typical solvent. A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : The eluting strength of a solvent is primarily related to how strongly it adsorbs onto the adsorbent and because typical adsorbents are. As with crystallization, solvent mixtures can be used to achieve. Tlc Solvent Polarity.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Tlc Solvent Polarity A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up the plate (equation 3). In all forms of chromatography, samples equilibrate. Tlc Solvent Polarity.
From mungfali.com
Solvent Polarity Chart Tlc Solvent Polarity High polarity solvents will do. Low polarity solvents will cause the spots to move more slowly. In all forms of chromatography, samples equilibrate between stationary and mobile phases. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or solvent mixture (the eluent ) that rises up. Tlc Solvent Polarity.