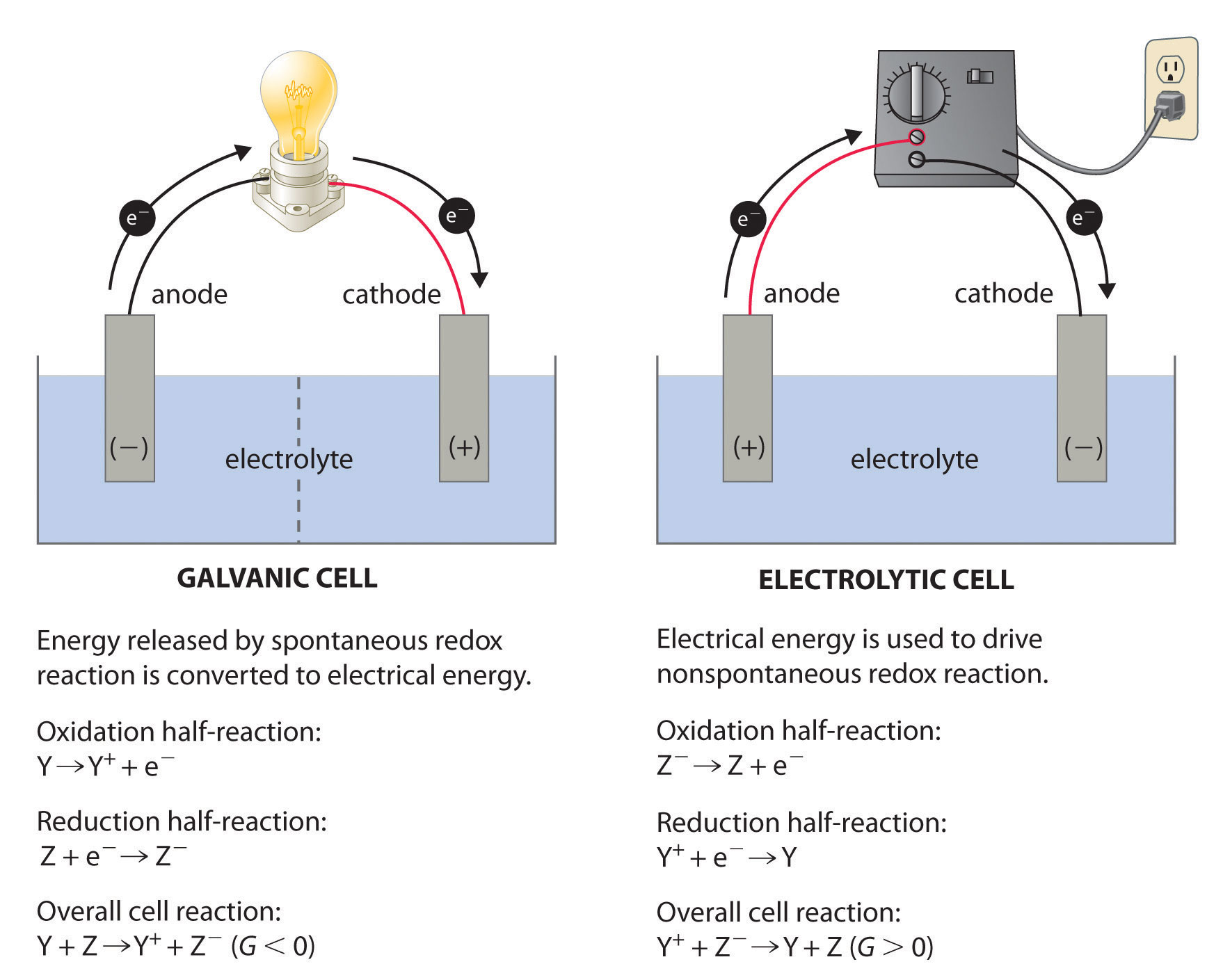
What Is The Difference Between Electrochemical Cell And Electrolytic Cell . An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrolytic cell is a form. In an electrochemical cell, half cells are placed in different containers and connected. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. The main differences are outlined below: However, there are also striking differences between the two cells. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and.
from webmis.highland.cc.il.us
An electrolytic cell is a form. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. In an electrochemical cell, half cells are placed in different containers and connected. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. The main differences are outlined below:
Describing Electrochemical Cells
What Is The Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. The main differences are outlined below: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In an electrochemical cell, half cells are placed in different containers and connected. An electrolytic cell is a form. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. However, there are also striking differences between the two cells. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy.
From stoplearn.com
Electrochemical Cells 2023 What Is The Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an electrochemical cell, half cells are placed in different containers and connected. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrolytic cell is a form. When an. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.thoughtco.com
Electrochemical Cell Definition What Is The Difference Between Electrochemical Cell And Electrolytic Cell A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. The main differences are outlined below: An electrolytic cell is a form. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In summary, electrochemical cells convert chemical energy into electrical energy. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From askfilo.com
These are of two types Electrochemical cell and Electrolytic cell. Sr. N.. What Is The Difference Between Electrochemical Cell And Electrolytic Cell In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrolytic cell is a form. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. The main differences are outlined below: A galvanic cell (left) transforms. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is The Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. In an electrochemical cell, half cells are placed in different containers and connected. The main. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube What Is The Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. The main differences are outlined below: In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrolytic cell is a form.. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. However, there are also striking differences between the two cells. An electrolytic cell is a form. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells What Is The Difference Between Electrochemical Cell And Electrolytic Cell In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. In an electrochemical cell, half cells are placed in different containers and connected. The most significant difference between an electrolytic cell and. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical What Is The Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: An electrolytic cell is a form. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. The most significant difference between an electrolytic cell and an. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. The main differences are outlined below: A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrolytic cell is a form. The most significant difference between an electrolytic cell and an electrochemical cell is that an. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.com
Difference Between Electrochemical Cell and Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. The main differences are outlined below: In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. In an electrochemical cell, half cells are placed in different containers and connected. A galvanic cell (left) transforms the energy released by a. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is The Difference Between Electrochemical Cell And Electrolytic Cell In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. An electrochemical cell splits the oxidant and reductant in a manner that. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From mmerevise.co.uk
Electrochemical Cells MME What Is The Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. In an electrochemical cell, half cells are placed in different containers. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is The Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an electrochemical cell, half cells are placed in different containers and connected. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrolytic cell is a form. When an electric current is passed through the cell,. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Electrochemical Cell vs Electrolytic Cell Electrochemistry YouTube What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrolytic cell is a form. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. The. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii What Is The Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected. The main differences are outlined below: However, there are also striking differences between the two cells. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. The most significant difference between an electrolytic cell. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From quizizz.com
Electrolytic Cell 385 plays Quizizz What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. An electrolytic cell is a form. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However,. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrolytic cell is a form. However, there are also striking differences between the two cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. In summary, electrochemical cells convert chemical energy. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.pt
Galvanic Cell and Electrolytic Cell Electrochemical Cells Teaching What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrolytic cell is a form. However, there are also striking differences between the two cells. When an electric current is passed. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. In an electrochemical cell, half cells are placed in different containers and connected. An electrolytic cell is a form. An electrochemical cell. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrolytic cell is a form. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and.. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube What Is The Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In an electrochemical cell, half cells are placed in different containers and connected. When an electric current is passed through the cell, ions migrate towards the electrodes, where. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
DIFFERENCE BETWEEN ELECTROLYTIC CELL & ELECTROCHEMICAL CELL YouTube What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrolytic cell is a form. The main differences are outlined below: The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrochemical cell splits the oxidant and reductant. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and What Is The Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. In an electrochemical cell, half cells are placed in different containers and connected. The main differences are outlined below: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From narodnatribuna.info
Electrolysis Electrolytic Cells And Electrolysis Process What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. In an electrochemical cell, half cells are placed in different containers and connected. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. The main differences are outlined below: The. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is The Difference Between Electrochemical Cell And Electrolytic Cell A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. The main differences are outlined below: However, there are also striking differences between the two cells. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. In an electrochemical cell, half. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. When an electric current is passed through the cell, ions migrate towards the electrodes, where. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.es
What are the differences between Galvanic cell and Electrolytic cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From www.bajeczneobrazy.pl
Electrolytic cell infographic diagram with components including anode What Is The Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. When an electric current is passed through the cell, ions migrate towards the electrodes, where they. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrolytic cell is a form. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. In an electrochemical cell, half cells are placed in different containers and connected. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrochemical. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From webmis.highland.cc.il.us
Describing Electrochemical Cells What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. In summary, electrochemical cells convert chemical energy into electrical energy through spontaneous redox reactions, while electrolytic. An electrolytic cell is a form. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.
From overallscience.com
Differences between electrolytic cell and voltaic cell Overall Science What Is The Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell is a system that uses spontaneous chemical reactions to generate electrical energy. However, there are also striking differences between the two cells. In an electrochemical cell, half cells are placed in different containers and connected. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the. What Is The Difference Between Electrochemical Cell And Electrolytic Cell.