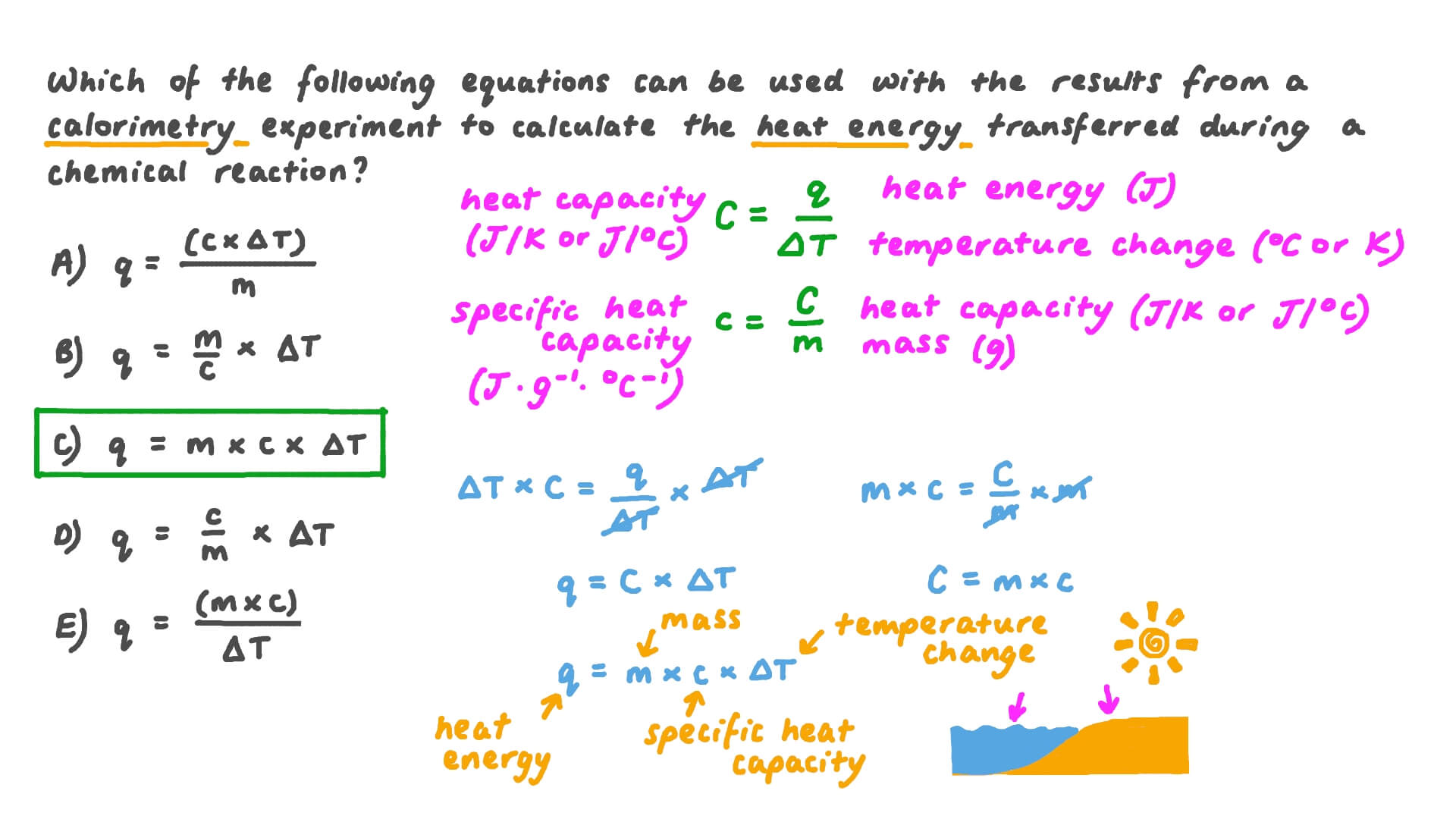Calorimetry Equations . See solved examples of calorimetry problems and the concept of heat of combustion. Find out how to calculate heat capacity and use calorimetry to measure heat. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Learn how to use ideal and real. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how to calculate and interpret heat and related properties using typical. Explore different types of calorimeters, endothermic. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. To do so, the heat is exchanged with a calibrated object. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques.
from
Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Explore different types of calorimeters, endothermic. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find out how to calculate heat capacity and use calorimetry to measure heat. To do so, the heat is exchanged with a calibrated object. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Find out how to calculate and interpret heat and related properties using typical. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry.
Calorimetry Equations Find out how to calculate heat capacity and use calorimetry to measure heat. Find out how to calculate heat capacity and use calorimetry to measure heat. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. To do so, the heat is exchanged with a calibrated object. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Explore different types of calorimeters, endothermic. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use ideal and real. Find out how to calculate and interpret heat and related properties using typical. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. See solved examples of calorimetry problems and the concept of heat of combustion. Calorimetry is used to measure amounts of heat transferred to or from a substance.
From chem.libretexts.org
3.4 Calorimetry Chemistry LibreTexts Calorimetry Equations See solved examples of calorimetry problems and the concept of heat of combustion. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use calorimetry to measure enthalpy changes in chemical. Calorimetry Equations.
From
Calorimetry Equations Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Find out how to calculate and interpret heat and related properties using typical. Learn how to measure the heat transfer in. Calorimetry Equations.
From
Calorimetry Equations Find out how to calculate heat capacity and use calorimetry to measure heat. Find out how to calculate and interpret heat and related properties using typical. To do so, the heat is exchanged with a calibrated object. Learn how to use ideal and real. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. See solved examples of calorimetry problems and the concept of heat of combustion. Find out how to calculate heat capacity and use calorimetry to measure heat. To do so, the heat is exchanged with a calibrated object. Learn how to measure the amount. Calorimetry Equations.
From
Calorimetry Equations See solved examples of calorimetry problems and the concept of heat of combustion. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. To do so, the heat is exchanged with a calibrated object. Explore different types of calorimeters, endothermic. Calorimetry is. Calorimetry Equations.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Equations Find out how to calculate and interpret heat and related properties using typical. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. See solved examples of calorimetry problems and the concept. Calorimetry Equations.
From
Calorimetry Equations Learn how to use ideal and real. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. To do so, the heat is exchanged with a calibrated object. Find out how to calculate. Calorimetry Equations.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Equations Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find out how to calculate and interpret heat and related properties using typical. Find out how to calculate heat capacity and use calorimetry to measure heat. Explore different types of calorimeters, endothermic.. Calorimetry Equations.
From
Calorimetry Equations Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure the heat transfer in chemical reactions using calorimeter and. Calorimetry Equations.
From
Calorimetry Equations To do so, the heat is exchanged with a calibrated object. Find out how to calculate and interpret heat and related properties using typical. Explore different types of calorimeters, endothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to measure the heat transfer in. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. To do so, the heat is exchanged with a calibrated object. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Find out how to calculate heat capacity and use calorimetry to measure heat. Calorimetry is used to. Calorimetry Equations.
From
Calorimetry Equations Find out how to calculate heat capacity and use calorimetry to measure heat. Find out how to calculate and interpret heat and related properties using typical. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. To do so,. Calorimetry Equations.
From www.youtube.com
Calorimetry Calculate Specific Heat YouTube Calorimetry Equations Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. To do so, the heat is exchanged with a calibrated object. Learn how to use calorimetry to measure enthalpy changes in chemical processes using. Calorimetry Equations.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimetry Equations Find out how to calculate heat capacity and use calorimetry to measure heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how to calculate and interpret heat and related properties using typical. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to measure the amount of. Calorimetry Equations.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Equations Find out how to calculate and interpret heat and related properties using typical. Find out how to calculate heat capacity and use calorimetry to measure heat. Explore different types of calorimeters, endothermic. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Calorimetry is an application of the first law of thermodynamics to. Calorimetry Equations.
From
Calorimetry Equations Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to use ideal and real. See solved examples of calorimetry problems and the concept of heat of combustion. To do. Calorimetry Equations.
From
Calorimetry Equations See solved examples of calorimetry problems and the concept of heat of combustion. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explore different types of calorimeters, endothermic. Learn how to measure the amount of heat involved in. Calorimetry Equations.
From
Calorimetry Equations Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Explore different types of calorimeters, endothermic. Learn about heat as a. Calorimetry Equations.
From
Calorimetry Equations To do so, the heat is exchanged with a calibrated object. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Explore different types of calorimeters, endothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances.. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Find out how to calculate heat capacity and use calorimetry to measure heat. Explore different types of calorimeters, endothermic. To do so, the heat is exchanged with a calibrated object. Learn. Calorimetry Equations.
From
Calorimetry Equations Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure the amount of heat. Calorimetry Equations.
From
Calorimetry Equations Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. To do so, the heat is exchanged with a calibrated object. Learn how to measure and calculate heat and enthalpy changes. Calorimetry Equations.
From www.chegg.com
Solved 10.oants Label each variable in the given calorimetry Calorimetry Equations Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Calorimetry is used to measure. Calorimetry Equations.
From
Calorimetry Equations Explore different types of calorimeters, endothermic. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Explore different types of calorimeters, endothermic. Find out how to calculate and interpret heat and related properties using typical. Calorimetry is used to measure amounts of heat transferred to. Calorimetry Equations.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Equations Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to use ideal and real. Explore different types of calorimeters, endothermic. To do so, the heat is exchanged with a calibrated object. Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Calorimetry is an application of the first law. Calorimetry Equations.
From
Calorimetry Equations Learn how to use ideal and real. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Find out how to calculate and interpret heat and related properties using typical. Calorimetry is used to measure amounts of. Calorimetry Equations.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Calorimetry Equations Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Find out how to calculate heat. Calorimetry Equations.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Equations Find out how to calculate and interpret heat and related properties using typical. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. Learn about heat as a form of energy transfer, internal energy, and. Calorimetry Equations.
From
Calorimetry Equations Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Find out how to calculate heat capacity and use calorimetry to measure heat. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to use ideal and real. Explore different types of calorimeters, endothermic. Calorimetry is used to measure. Calorimetry Equations.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Equations Learn how to measure the amount of heat involved in a chemical or physical process using calorimetry. Learn about heat as a form of energy transfer, internal energy, and the mechanical equivalent of heat. Find out how to calculate heat capacity and use calorimetry to measure heat. See solved examples of calorimetry problems and the concept of heat of combustion.. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure the amount of heat involved in a physical or chemical reaction using calorimeters and the calorimetry equation. To do so, the heat is exchanged with a calibrated object. See solved examples of calorimetry problems and the concept of heat of combustion. Learn how to measure the amount of heat involved in a chemical or physical process. Calorimetry Equations.
From
Calorimetry Equations To do so, the heat is exchanged with a calibrated object. Explore different types of calorimeters, endothermic. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Find out how to calculate and interpret heat and related properties using typical. Learn how to measure the amount of heat involved in a physical or chemical. Calorimetry Equations.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Equations Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to use ideal and real. See solved examples of calorimetry problems and the concept of heat of combustion. Explore different types of calorimeters, endothermic. Find out how to calculate heat capacity and use calorimetry to measure heat. Learn about heat as a form of energy. Calorimetry Equations.
From
Calorimetry Equations Learn how to measure the heat transfer in chemical reactions using calorimeter and calorimetry formula. Find out how to calculate and interpret heat and related properties using typical. Learn how to use ideal and real. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved. Calorimetry Equations.