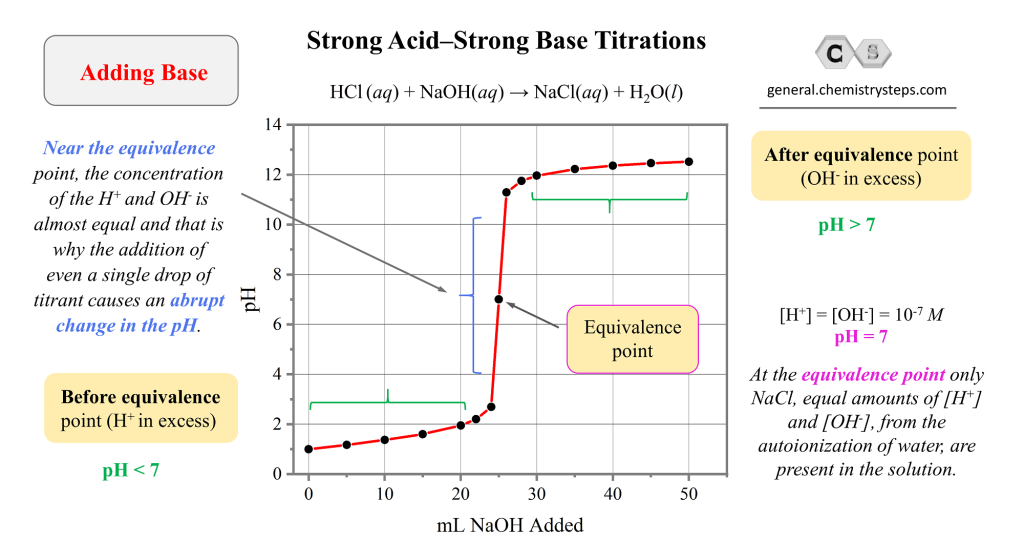
Equivalence Point During The Titration . To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence points are not always ph 7. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point → moles of alkali = moles of acid. This is also known as the equivalence point and this is. The equivalence point is halfway the vertical region of the curve. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it.
from general.chemistrysteps.com
To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence points are not always ph 7. This is also known as the equivalence point and this is. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Equivalence point → moles of alkali = moles of acid. The equivalence point of a titration.
Strong AcidStrong Base Titrations Chemistry Steps
Equivalence Point During The Titration Equivalence points are not always ph 7. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Equivalence points are not always ph 7. In other words, while titrating, it. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This is also known as the equivalence point and this is. The equivalence point is halfway the vertical region of the curve. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point → moles of alkali = moles of acid. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Equivalence Point During The Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. At the equivalence point in a neutralization, the moles of acid are equal to. Equivalence Point During The Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point During The Titration In other words, while titrating, it. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This is also known as the equivalence point and this is. The equivalence point is. Equivalence Point During The Titration.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Equivalence Point During The Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Equivalence point → moles of alkali = moles of acid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence points are not always ph 7. The equivalence point is halfway the. Equivalence Point During The Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Equivalence Point During The Titration In other words, while titrating, it. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This is also known as the equivalence point. Equivalence Point During The Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point During The Titration The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. In other words, while titrating, it. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point is. Equivalence Point During The Titration.
From www.numerade.com
SOLVEDWhat is a titration? What is the equivalence point? Equivalence Point During The Titration The equivalence point of a titration. Equivalence point → moles of alkali = moles of acid. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the. Equivalence Point During The Titration.
From philschatz.com
AcidBase Titrations · Chemistry Equivalence Point During The Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is halfway the vertical region of the curve. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. At the equivalence point in a neutralization, the moles. Equivalence Point During The Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Equivalence Point During The Titration Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Equivalence Point During The Titration.
From www.gauthmath.com
Solved What does equivalence point refer to during a titration? A. The Equivalence Point During The Titration The equivalence point of a titration. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. To evaluate the relationship between a titration’s equivalence point and its end. Equivalence Point During The Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Equivalence Point During The Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. This is also known as the equivalence point and this is. In other words, while titrating, it. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have. Equivalence Point During The Titration.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Equivalence Point During The Titration In other words, while titrating, it. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The equivalence point is halfway the vertical region of the curve. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed.. Equivalence Point During The Titration.
From www.youtube.com
Finding equivalence point for Monoprotic acid YouTube Equivalence Point During The Titration The equivalence point of a titration. Equivalence points are not always ph 7. In other words, while titrating, it. The equivalence point is halfway the vertical region of the curve. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. This is also known as the equivalence. Equivalence Point During The Titration.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Equivalence Point During The Titration The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. Equivalence point → moles of alkali = moles of acid. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. In. Equivalence Point During The Titration.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby Equivalence Point During The Titration In other words, while titrating, it. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence points are not always ph 7. This is also known as the equivalence point and this is. Equivalence point → moles of alkali = moles of acid. The equivalence point of. Equivalence Point During The Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Equivalence Point During The Titration Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. Equivalence point → moles of alkali = moles of acid. The equivalence point is halfway the vertical region of the curve. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Equivalence Point During The Titration.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Equivalence Point During The Titration In other words, while titrating, it. This is also known as the equivalence point and this is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. To. Equivalence Point During The Titration.
From byjus.com
The graph of pH during the titration of NaOH and HCl Equivalence Point During The Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. Equivalence points are not always ph 7. To evaluate the relationship between a titration’s equivalence point and its end. Equivalence Point During The Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point During The Titration This is also known as the equivalence point and this is. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In other words, while titrating, it. Equivalence points are not always ph 7. The equivalence point of a titration. The equivalence point of a chemical reaction is the. Equivalence Point During The Titration.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Equivalence Point During The Titration In other words, while titrating, it. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point is halfway the vertical region of the curve. The equivalence point. Equivalence Point During The Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point During The Titration Equivalence points are not always ph 7. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. This is also known as the equivalence point and this is. The equivalence point is halfway the vertical region of the curve. To evaluate the relationship between a titration’s equivalence point and its end. Equivalence Point During The Titration.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point During The Titration In other words, while titrating, it. The equivalence point is halfway the vertical region of the curve. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence points are not always ph 7. At the equivalence point in a neutralization, the moles of acid are equal to. Equivalence Point During The Titration.
From mavink.com
What Is The Equivalence Point Of A Titration Equivalence Point During The Titration Equivalence point → moles of alkali = moles of acid. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence points are not always ph 7.. Equivalence Point During The Titration.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Equivalence Point During The Titration This is also known as the equivalence point and this is. Equivalence points are not always ph 7. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and. Equivalence Point During The Titration.
From mungfali.com
Equivalence Points On Titration Graph Equivalence Point During The Titration Equivalence point → moles of alkali = moles of acid. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence points are not always ph 7. This is also known as the. Equivalence Point During The Titration.
From klaakyfwn.blob.core.windows.net
Dynamic Equivalence Point Titration at Sonja Hogan blog Equivalence Point During The Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Equivalence points are not always ph 7. The equivalence point of a titration. In other words, while titrating, it. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant,. Equivalence Point During The Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Equivalence Point During The Titration The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point → moles of alkali = moles of acid. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In other words, while titrating,. Equivalence Point During The Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Equivalence Point During The Titration This is also known as the equivalence point and this is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is halfway the vertical region of the curve. Equivalence point → moles of alkali = moles of acid. The equivalence point of a titration. Equivalence points are. Equivalence Point During The Titration.
From klaakyfwn.blob.core.windows.net
Dynamic Equivalence Point Titration at Sonja Hogan blog Equivalence Point During The Titration Equivalence point → moles of alkali = moles of acid. Equivalence points are not always ph 7. The equivalence point of a titration. In other words, while titrating, it. This is also known as the equivalence point and this is. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable. Equivalence Point During The Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Equivalence Point During The Titration To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The equivalence point is halfway the vertical region of the curve. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. At the equivalence point. Equivalence Point During The Titration.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Equivalence Point During The Titration In other words, while titrating, it. This is also known as the equivalence point and this is. The equivalence point of a titration. Equivalence point → moles of alkali = moles of acid. The equivalence point is halfway the vertical region of the curve. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Equivalence Point During The Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point During The Titration Equivalence points are not always ph 7. Equivalence point → moles of alkali = moles of acid. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence. Equivalence Point During The Titration.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog Equivalence Point During The Titration This is also known as the equivalence point and this is. Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the. Equivalence Point During The Titration.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Equivalence Point During The Titration This is also known as the equivalence point and this is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. Equivalence points are not always ph 7. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if. Equivalence Point During The Titration.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Equivalence Point During The Titration The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence point → moles of alkali = moles of acid. The equivalence point is halfway the vertical region of the curve. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only. Equivalence Point During The Titration.
From www.numerade.com
SOLVED The diagrams below represent the change in the composition of a Equivalence Point During The Titration To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence points are not always ph 7. Equivalence point → moles of alkali = moles of acid. Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the. Equivalence Point During The Titration.