Hard Water Analysis Lab Report . In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. To learn the cause and effects of hard water; Gravimetric analysis is where the amount of analyte can be. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. Waters softer than 30 to 50 mg/l may be corrosive to piping. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. Scale builds up in pipes, on. Calcite crystals, which results in scale formation. When hard water evaporates, it leaves behind crystals, mainly. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties of.
from braukaiser.com
Waters softer than 30 to 50 mg/l may be corrosive to piping. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. Calcite crystals, which results in scale formation. Gravimetric analysis is where the amount of analyte can be. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. Scale builds up in pipes, on. When hard water evaporates, it leaves behind crystals, mainly. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. To learn the cause and effects of hard water;
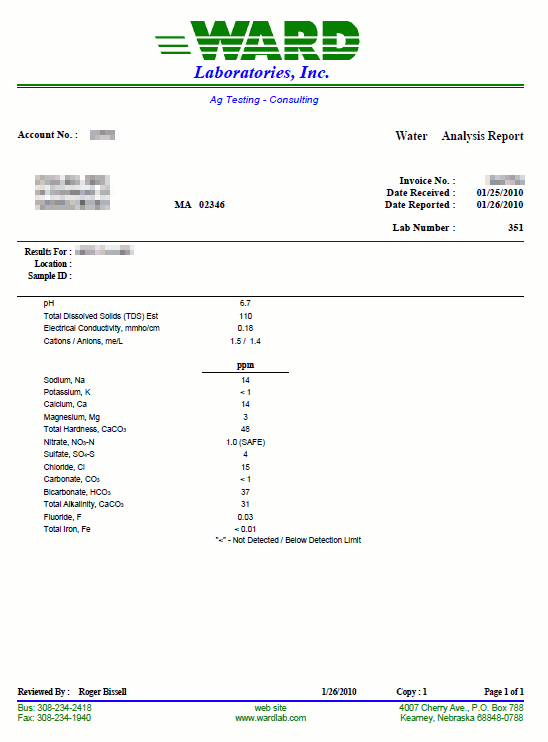
How to read a water report German brewing and more
Hard Water Analysis Lab Report Calcite crystals, which results in scale formation. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. When hard water evaporates, it leaves behind crystals, mainly. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Scale builds up in pipes, on. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. Calcite crystals, which results in scale formation. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties of. Gravimetric analysis is where the amount of analyte can be. To learn the cause and effects of hard water; Waters softer than 30 to 50 mg/l may be corrosive to piping. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents.
From www.slideserve.com
PPT Water Hardness Determination with EDTA PowerPoint Presentation Hard Water Analysis Lab Report When hard water evaporates, it leaves behind crystals, mainly. Gravimetric analysis is where the amount of analyte can be. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. In the hard. Hard Water Analysis Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Hard Water Analysis Date Hard Water Analysis Lab Report Waters softer than 30 to 50 mg/l may be corrosive to piping. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties of. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical. Hard Water Analysis Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Hard Water Analysis Dorta Hard Water Analysis Lab Report When hard water evaporates, it leaves behind crystals, mainly. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. Scale builds up in pipes, on. Gravimetric analysis is where the amount of analyte can be. Waters softer than 30 to 50 mg/l may be corrosive to piping. Try. Hard Water Analysis Lab Report.
From studylib.net
Laboratory Microbiological Water Sample Report Hard Water Analysis Lab Report Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties of. Calcite crystals, which results in scale formation. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium. Hard Water Analysis Lab Report.
From www.scientific.net
Research on the Determination of Total Hardness in Drinking Water by Hard Water Analysis Lab Report Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Scale builds up in pipes, on. Calcite crystals, which results in scale formation. When hard water evaporates, it leaves behind crystals, mainly. Gravimetric analysis is where the amount of analyte can. Hard Water Analysis Lab Report.
From www.researchgate.net
1. LABORATORY WATER QUALITY ANALYSIS REPORT Download Table Hard Water Analysis Lab Report In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Scale builds up in pipes, on. When hard water evaporates, it leaves behind crystals, mainly.. Hard Water Analysis Lab Report.
From paperap.com
Pond Water Lab Report Analysis Free Essay Example Hard Water Analysis Lab Report The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Waters softer than 30 to 50 mg/l may be corrosive to piping. In this experiment. Hard Water Analysis Lab Report.
From www.studocu.com
Water Quality LAB Report EC2206A7B ENVIRONMENTAL ENGINEERING Hard Water Analysis Lab Report Scale builds up in pipes, on. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. In this experiment, complexometric. Hard Water Analysis Lab Report.
From www.studocu.com
Properties of Water Lab Report Properties of Water Lab Report Hard Water Analysis Lab Report In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Calcite crystals, which results in scale formation. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties. Hard Water Analysis Lab Report.
From www.slideshare.net
Lab5 determination of hardness of water Hard Water Analysis Lab Report Calcite crystals, which results in scale formation. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate. Hard Water Analysis Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Hard Water Analysis Data Hard Water Analysis Lab Report To learn the cause and effects of hard water; In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Gravimetric analysis is where the amount of analyte can be. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling. Hard Water Analysis Lab Report.
From exonzmvvd.blob.core.windows.net
Water Hardness Test Reaction at Anne Howard blog Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. When hard water evaporates, it. Hard Water Analysis Lab Report.
From www.slideshare.net
Lab5 determination of hardness of water Hard Water Analysis Lab Report In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. Scale builds up in pipes, on. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. When. Hard Water Analysis Lab Report.
From www.scribd.com
Water Hardness Lab Report Titration Chemistry Hard Water Analysis Lab Report Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. The hardness of good quality water should not exceed 250. Hard Water Analysis Lab Report.
From www.studocu.com
LAB Report Water Quality ( Drinking Water) Result AND DATA Analysis Hard Water Analysis Lab Report The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Scale builds up in pipes, on. Analysis of hard water introduction the purpose of this. Hard Water Analysis Lab Report.
From braukaiser.com
How to read a water report German brewing and more Hard Water Analysis Lab Report Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. To determine the hardness of a water sample the following. Hard Water Analysis Lab Report.
From es-mx.watergroup.com
Formulario de análisis del agua WaterGroup Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Calcite crystals, which results in scale formation. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate. Hard Water Analysis Lab Report.
From www.scribd.com
Water Testing Lab Report PDF Precipitation (Chemistry) Sodium Hard Water Analysis Lab Report Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Gravimetric analysis is where the amount of analyte can be. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3.. Hard Water Analysis Lab Report.
From www.chegg.com
HARD WATER ANALYSIS LAB OBSERVATIONS TOTAL HARDNESS Hard Water Analysis Lab Report The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. To learn the cause and effects of hard water; Waters softer than 30 to 50 mg/l may be corrosive to piping. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. Gravimetric analysis is where the amount. Hard Water Analysis Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Hard Water Analysis Date Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. Calcite crystals, which results in scale formation. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. When hard water evaporates, it leaves behind crystals, mainly. Scale. Hard Water Analysis Lab Report.
From www.slideshare.net
Lab5 determination of hardness of water Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Analysis of hard water introduction the purpose of this laboratory exercise is to demonstrate the properties of. Try this experiment with your students to measure the hardness of different water samples and investigate the. Hard Water Analysis Lab Report.
From studylib.net
LAB HARD WATER ANALYSIS Hard Water Analysis Lab Report To learn the cause and effects of hard water; Scale builds up in pipes, on. Calcite crystals, which results in scale formation. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Analysis of hard water introduction the purpose of this. Hard Water Analysis Lab Report.
From www.researchgate.net
Water analysis report. Download Table Hard Water Analysis Lab Report In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. Gravimetric analysis is where the amount of analyte can be. When hard water evaporates, it leaves behind crystals, mainly. In this. Hard Water Analysis Lab Report.
From www.studocu.com
Domestic Use water Analysis lab Report 07/07/ Domestic Water Use Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. In this experiment you will standardize a solution of edta by titration. Hard Water Analysis Lab Report.
From www.scribd.com
Water Analysis Lab Report PDF Alkalinity Water Purification Hard Water Analysis Lab Report When hard water evaporates, it leaves behind crystals, mainly. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. To learn the cause and effects of hard water; Gravimetric analysis is where the amount of analyte can be. In this experiment you will standardize a solution of edta by titration against a standard solution. Hard Water Analysis Lab Report.
From www.chegg.com
CHEMISTRY HARDNESS OF WATER LAB REPORT PLEASE HELP IM Hard Water Analysis Lab Report In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. To learn the cause and effects of hard water; Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a.. Hard Water Analysis Lab Report.
From www.studocu.com
Chem Lab Report 8 Water Hardness Chem Lab Report 8 Water Hardness Hard Water Analysis Lab Report Calcite crystals, which results in scale formation. Scale builds up in pipes, on. To learn the cause and effects of hard water; The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. When hard water evaporates, it leaves behind crystals, mainly. In the hard water lab, the calcium concentration of loyola’s water was determined. Hard Water Analysis Lab Report.
From www.chegg.com
HARD WATER ANALYSIS LAB OBSERVATIONS TOTAL HARDNESS Hard Water Analysis Lab Report When hard water evaporates, it leaves behind crystals, mainly. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. Scale builds up in pipes, on. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where. Hard Water Analysis Lab Report.
From www.chegg.com
hardness of water lab report .. I don't know ehat Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. Calcite crystals, which results in scale formation. Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. In this experiment you will standardize a solution of edta by titration against a. Hard Water Analysis Lab Report.
From www.chegg.com
HARD WATER ANALYSIS REPORT SHEET PART A Hard Water Analysis Lab Report Try this experiment with your students to measure the hardness of different water samples and investigate the effect of boiling hard water this is a student practical, where a. Waters softer than 30 to 50 mg/l may be corrosive to piping. Gravimetric analysis is where the amount of analyte can be. In this experiment you will standardize a solution of. Hard Water Analysis Lab Report.
From www.chegg.com
Solved Experiment 21 Report Sheet Hard Water Analysis Data Hard Water Analysis Lab Report In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. Waters softer than 30 to 50 mg/l may be corrosive to piping. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. In the hard water lab, the calcium concentration of. Hard Water Analysis Lab Report.
From www.water-rightgroup.com
Try This Easy DIY Test for Hard Water WaterRight Hard Water Analysis Lab Report Calcite crystals, which results in scale formation. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. To determine the hardness of a water sample the following techniques are used in the experimental. Hard Water Analysis Lab Report.
From sprayers101.com
How to interpret a water quality test result Sprayers 101 Hard Water Analysis Lab Report Scale builds up in pipes, on. When hard water evaporates, it leaves behind crystals, mainly. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Waters softer than 30 to 50 mg/l may be corrosive to piping. To determine the hardness of a water sample the following techniques are used in the experimental procedure:.. Hard Water Analysis Lab Report.
From www.slideshare.net
Lab5 determination of hardness of water Hard Water Analysis Lab Report In this experiment, complexometric titration is used to determine the hardness of water which is defined by the unit milligrams of calcium. The hardness of good quality water should not exceed 250 mg/l measured as calcium carbonate equivalents. Gravimetric analysis is where the amount of analyte can be. To learn the cause and effects of hard water; In the hard. Hard Water Analysis Lab Report.
From studylib.net
Analysis of Hard Water Lab Hard Water Analysis Lab Report Gravimetric analysis is where the amount of analyte can be. To determine the hardness of a water sample the following techniques are used in the experimental procedure:. When hard water evaporates, it leaves behind crystals, mainly. In the hard water lab, the calcium concentration of loyola’s water was determined using gravimetrical analysis. To learn the cause and effects of hard. Hard Water Analysis Lab Report.