Standard Enthalpy Of Formation Of H2S(G) . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. View plot requires a javascript / html 5 canvas capable browser. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Hydrogen sulfide, h2s, is a. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. The correlation coefficients are obtained by. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy.
from www.numerade.com
H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. Hydrogen sulfide, h2s, is a. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: The correlation coefficients are obtained by. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. View plot requires a javascript / html 5 canvas capable browser. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy.
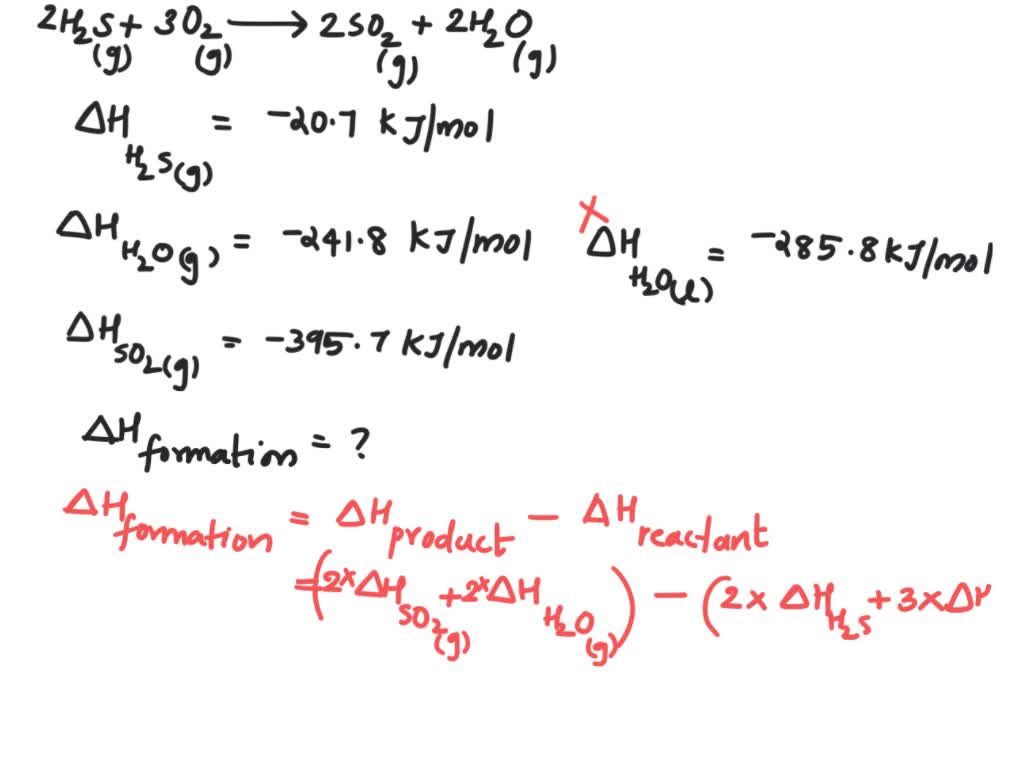
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2
Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. View plot requires a javascript / html 5 canvas capable browser. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. The correlation coefficients are obtained by. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. Hydrogen sulfide, h2s, is a.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Enthalpy Of Formation Of H2S(G) H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety. Standard Enthalpy Of Formation Of H2S(G).
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Of H2S(G) Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. H° = standard enthalpy (kj/mol) s° =. Standard Enthalpy Of Formation Of H2S(G).
From www.slideserve.com
PPT Example using enthalpy of combustion and formation PowerPoint Standard Enthalpy Of Formation Of H2S(G) The correlation coefficients are obtained by. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Top. Standard Enthalpy Of Formation Of H2S(G).
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Standard Enthalpy Of Formation Of H2S(G) Hydrogen sulfide, h2s, is a. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: The correlation coefficients are obtained by. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid,. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Use standard enthalpies of formation to calculate the standard Standard Enthalpy Of Formation Of H2S(G) H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s,. Standard Enthalpy Of Formation Of H2S(G).
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of H2S(G) S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Hydrogen sulfide, h2s, is a. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: The standard enthalpy of. Standard Enthalpy Of Formation Of H2S(G).
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Of H2S(G) 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The correlation coefficients are obtained by. S° = a*ln(t) + b*t + c*t 2 /2. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Of H2S(G) Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. View plot requires a javascript / html 5 canvas capable browser. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Of H2S(G).
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Of H2S(G) Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Of H2S(G) Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. The correlation coefficients are obtained by. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Sulfuric Acid Standard Enthalpy Of Formation Of H2S(G) 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Calculate the heat of combustion for the reaction given below Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p =. Standard Enthalpy Of Formation Of H2S(G).
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Hydrogen sulfide, h2s, is a. 136 rows standard enthalpy change of formation (data table) these tables. Standard Enthalpy Of Formation Of H2S(G).
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Of H2S(G) Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Hydrogen sulfide, h2s, is a. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. View. Standard Enthalpy Of Formation Of H2S(G).
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Of H2S(G) S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. H° = standard. Standard Enthalpy Of Formation Of H2S(G).
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Of H2S(G) 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Formation Of H2S(G) S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Of H2S(G).
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Enthalpy Of Formation Of H2S(G) The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and. Standard Enthalpy Of Formation Of H2S(G).
From solvedlib.com
Calculate enthalpy change for this reaction_Hydrogen … SolvedLib Standard Enthalpy Of Formation Of H2S(G) The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g). Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED A chemist measures the enthalpy change AH during the following Standard Enthalpy Of Formation Of H2S(G) 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The correlation coefficients are obtained by. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. S° = a*ln(t) + b*t + c*t 2 /2. Standard Enthalpy Of Formation Of H2S(G).
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Of H2S(G) Hydrogen sulfide, h2s, is a. View plot requires a javascript / html 5 canvas capable browser. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation Of H2S(G) S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Hydrogen sulfide, h2s, is a. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer gas and stink damp. The standard. Standard Enthalpy Of Formation Of H2S(G).
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The correlation coefficients are obtained by. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. S° = a*ln(t) + b*t + c*t 2 /2. Standard Enthalpy Of Formation Of H2S(G).
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of H2S(G) View plot requires a javascript / html 5 canvas capable browser. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° =. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Part 1 Calculate the deltaH for the reaction. Use the heat of Standard Enthalpy Of Formation Of H2S(G) 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The correlation coefficients are obtained by. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard. Standard Enthalpy Of Formation Of H2S(G).
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Of H2S(G) S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Hydrogen sulfide, h2s,. Standard Enthalpy Of Formation Of H2S(G).
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Of H2S(G) The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Hydrogen sulfide, h2s, is a. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° =. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p =. Standard Enthalpy Of Formation Of H2S(G).
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of H2S(G) Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Hydrogen sulfide, h2s, is a. Top. Standard Enthalpy Of Formation Of H2S(G).
From www.slideserve.com
PPT Chapter 17 Energy and Chemical Change PowerPoint Presentation Standard Enthalpy Of Formation Of H2S(G) Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Hydrogen sulfide, h2s, is a. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Of H2S(G).
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of H2S(G) View plot requires a javascript / html 5 canvas capable browser. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Chemical, physical and thermal properties of hydrogen sulfide, h 2 s, also called hydrosulfuric acid, sewer. Standard Enthalpy Of Formation Of H2S(G).
From hydrogengasgaosube.blogspot.com
Hydrogen Gas June 2017 Standard Enthalpy Of Formation Of H2S(G) 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The correlation coefficients are obtained by. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation Of H2S(G).
From www.numerade.com
SOLVED Given the standard enthalpies of formation for the table below Standard Enthalpy Of Formation Of H2S(G) The correlation coefficients are obtained by. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. View plot requires a javascript / html 5 canvas capable browser. H° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t = temperature (k) / 1000. Chemical, physical and thermal properties. Standard Enthalpy Of Formation Of H2S(G).
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID6274598 Standard Enthalpy Of Formation Of H2S(G) Hydrogen sulfide, h2s, is a. Top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Of H2S(G).
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Of H2S(G) The correlation coefficients are obtained by. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. S° = a*ln(t) + b*t + c*t 2 /2 + d*t 3 /3 − e/(2*t 2) + g c p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy.. Standard Enthalpy Of Formation Of H2S(G).