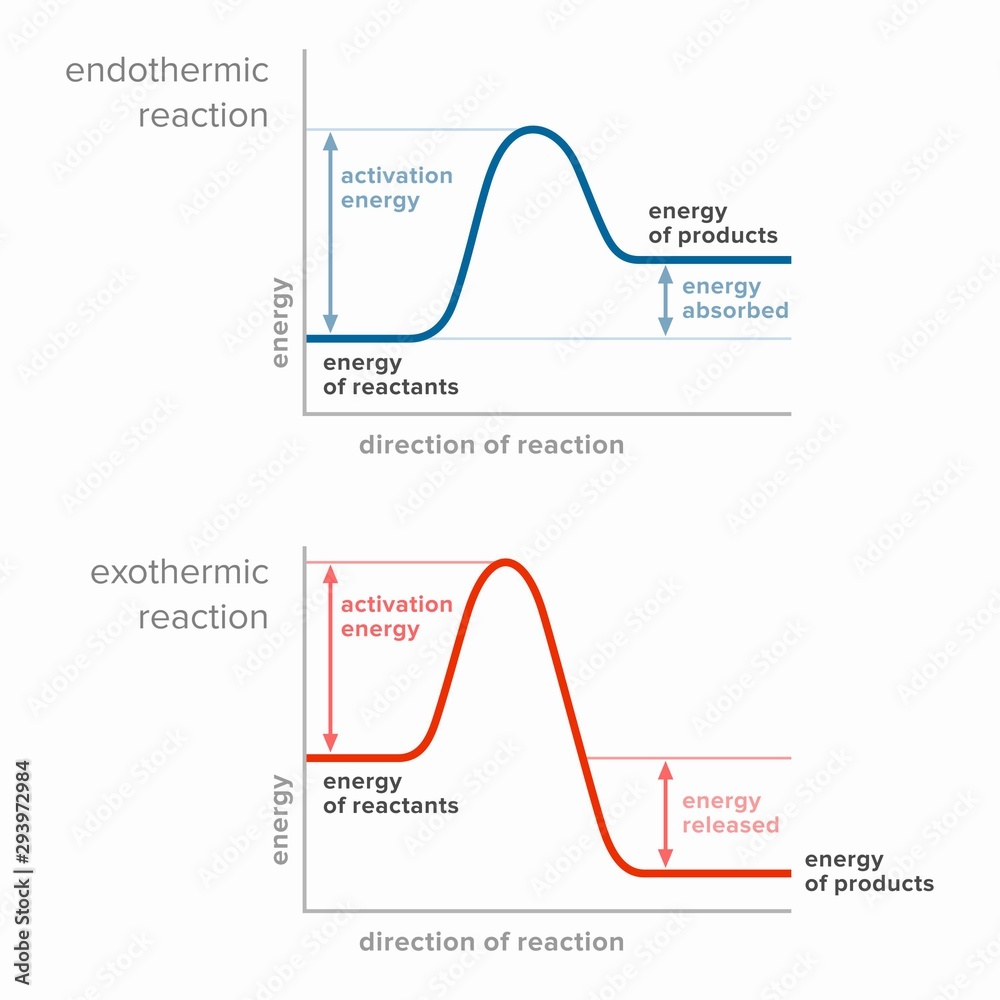
Endothermic Reaction Favoured By . Anther way to view endothermic reactions is that. The reaction between ethanoic acid and sodium carbonate. endothermic reaction examples. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. some examples of endothermic reactions are: the reactants transform into products. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. When temperature is the stress that affects a system at. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a system at equilibrium. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place.
from stock.adobe.com
They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. explain how temperature changes affect a system at equilibrium. the reactants transform into products. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. some examples of endothermic reactions are: endothermic reaction examples. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Anther way to view endothermic reactions is that.
Activation energy in endothermic and exothermic reactions. Stock
Endothermic Reaction Favoured By They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. some examples of endothermic reactions are: They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. the reactants transform into products. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a system at equilibrium. The reaction between ethanoic acid and sodium carbonate. Anther way to view endothermic reactions is that. endothermic reaction examples. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. When temperature is the stress that affects a system at.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Favoured By the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. the reactants transform into products. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. They absorb heat energy to overcome. Endothermic Reaction Favoured By.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Favoured By endothermic reaction examples. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. the reactants transform into products. endothermic and exothermic reactions can be thought of as having energy. Endothermic Reaction Favoured By.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Favoured By endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a system at equilibrium. the reactants transform into products. endothermic reaction examples. They absorb heat energy to overcome. Endothermic Reaction Favoured By.
From thechemistrynotes.com
Endothermic Reaction with Interesting Examples Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. endothermic and exothermic reactions can be thought of as having energy as either. Endothermic Reaction Favoured By.
From www.toppr.com
K an endoth endothermic chemical reaction is 10 atm backward reaction Endothermic Reaction Favoured By Anther way to view endothermic reactions is that. explain how temperature changes affect a system at equilibrium. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. endothermic reaction examples. some examples of endothermic reactions are: the reactants transform into products. in endothermic reactions, (\(δh>0\)) thermal energy is. Endothermic Reaction Favoured By.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Endothermic Reaction Favoured By They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. some examples of endothermic reactions are: Anther way to view endothermic reactions is that. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. The reaction between ethanoic acid and sodium carbonate. the reactants transform into products. endothermic. Endothermic Reaction Favoured By.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. the reactants transform into products. When temperature is the stress that affects a system at. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction. Endothermic Reaction Favoured By.
From present5.com
Chapter 1 1 Endothermic and Exothermic Reactions Endothermic Reaction Favoured By Anther way to view endothermic reactions is that. the reactants transform into products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The reaction between ethanoic acid and sodium carbonate. When temperature is the stress that affects a system at. When ammonium chloride (nh 4 cl). Endothermic Reaction Favoured By.
From circuiteracktiergotorg.z13.web.core.windows.net
How To Read Energy Diagrams Chemistry Endothermic Reaction Favoured By the reactants transform into products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reaction examples. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. They absorb heat energy to overcome the activation energy barrier that allows the. Endothermic Reaction Favoured By.
From slideplayer.com
Exothermic and Endothermic Reactions Oxidation Reactions ppt download Endothermic Reaction Favoured By When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Anther way to view endothermic reactions is that. When temperature is the stress that affects a system at. explain how temperature changes affect a system at equilibrium. the rate of the endothermic reaction is increased more than the rate of the backward reaction. Endothermic Reaction Favoured By.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Favoured By endothermic reaction examples. Anther way to view endothermic reactions is that. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. The reaction between ethanoic acid and sodium carbonate. When temperature is the stress that affects a system at. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. endothermic and. Endothermic Reaction Favoured By.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction Favoured By When temperature is the stress that affects a system at. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. explain how temperature changes affect a system at equilibrium. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an. Endothermic Reaction Favoured By.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. the reactants transform into products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. the rate of the endothermic reaction is increased more than the. Endothermic Reaction Favoured By.
From studylib.net
Lesson 7 Reaction profiles Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. They absorb heat energy to overcome the activation energy barrier that allows the reaction to. Endothermic Reaction Favoured By.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Favoured By Anther way to view endothermic reactions is that. When temperature is the stress that affects a system at. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. The reaction between ethanoic acid and sodium carbonate. the rate of the endothermic reaction. Endothermic Reaction Favoured By.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Favoured By endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. the reactants transform into products. some examples of endothermic reactions are: When temperature is the stress that affects a system at. They absorb heat. Endothermic Reaction Favoured By.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction Favoured By some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. endothermic reaction examples. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response. Endothermic Reaction Favoured By.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Favoured By the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. Anther way to view endothermic reactions is that. explain how temperature changes affect a system at equilibrium. The reaction between ethanoic acid and sodium carbonate. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. . Endothermic Reaction Favoured By.
From guideelsk5f.z4.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Reaction Favoured By some examples of endothermic reactions are: explain how temperature changes affect a system at equilibrium. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. Anther way to view endothermic reactions is that. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. endothermic. Endothermic Reaction Favoured By.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Favoured By explain how temperature changes affect a system at equilibrium. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reaction examples. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed. Endothermic Reaction Favoured By.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Favoured By When temperature is the stress that affects a system at. Anther way to view endothermic reactions is that. explain how temperature changes affect a system at equilibrium. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate.. Endothermic Reaction Favoured By.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Favoured By in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. The reaction between ethanoic acid and sodium carbonate. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. . Endothermic Reaction Favoured By.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Reaction Favoured By in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. the reactants transform into products. When temperature is the stress that affects a system at. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. some examples of endothermic reactions are: They absorb heat. Endothermic Reaction Favoured By.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Favoured By When temperature is the stress that affects a system at. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reaction examples. Anther way to view endothermic reactions is. Endothermic Reaction Favoured By.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction Favoured By explain how temperature changes affect a system at equilibrium. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. endothermic reaction examples. When temperature is the stress that affects a system at. Anther way to view endothermic reactions is that. the rate of the endothermic reaction is increased more than the rate of the backward. Endothermic Reaction Favoured By.
From learningschoolcouleemg.z4.web.core.windows.net
Exothermic And Endothermic Reactions Worksheets Answers Endothermic Reaction Favoured By the reactants transform into products. The reaction between ethanoic acid and sodium carbonate. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response. Endothermic Reaction Favoured By.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Favoured By When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. The reaction between ethanoic acid and sodium carbonate. Anther way to view. Endothermic Reaction Favoured By.
From slideplayer.com
Chapter 21 Types of Reactions. ppt download Endothermic Reaction Favoured By Anther way to view endothermic reactions is that. endothermic reaction examples. The reaction between ethanoic acid and sodium carbonate. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a. Endothermic Reaction Favoured By.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. When temperature is the stress that affects a system at. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a system at. Endothermic Reaction Favoured By.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Favoured By When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. When temperature is the stress that affects a system at. explain how temperature changes affect a system at equilibrium. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. The reaction between ethanoic. Endothermic Reaction Favoured By.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Favoured By When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. the reactants transform into products. some examples of endothermic reactions are: When temperature is the stress that affects a system at. in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. explain how temperature changes affect a system at equilibrium.. Endothermic Reaction Favoured By.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Favoured By They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. some examples of endothermic reactions are: endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes. Endothermic Reaction Favoured By.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Favoured By the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. the reactants transform into products. explain how temperature changes affect a system at equilibrium. in endothermic reactions, (\(δh>0\)) thermal energy is. Endothermic Reaction Favoured By.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction Favoured By When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. some examples of endothermic reactions are: endothermic reaction examples. the reactants transform into products. Anther way to view endothermic reactions is. Endothermic Reaction Favoured By.
From classzonesalicetum.z14.web.core.windows.net
Identify Endothermic And Exothermic Reactions Endothermic Reaction Favoured By The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: in endothermic reactions, (\(δh>0\)) thermal energy is absorbed via the reaction. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. the rate of the endothermic reaction is increased more than. Endothermic Reaction Favoured By.