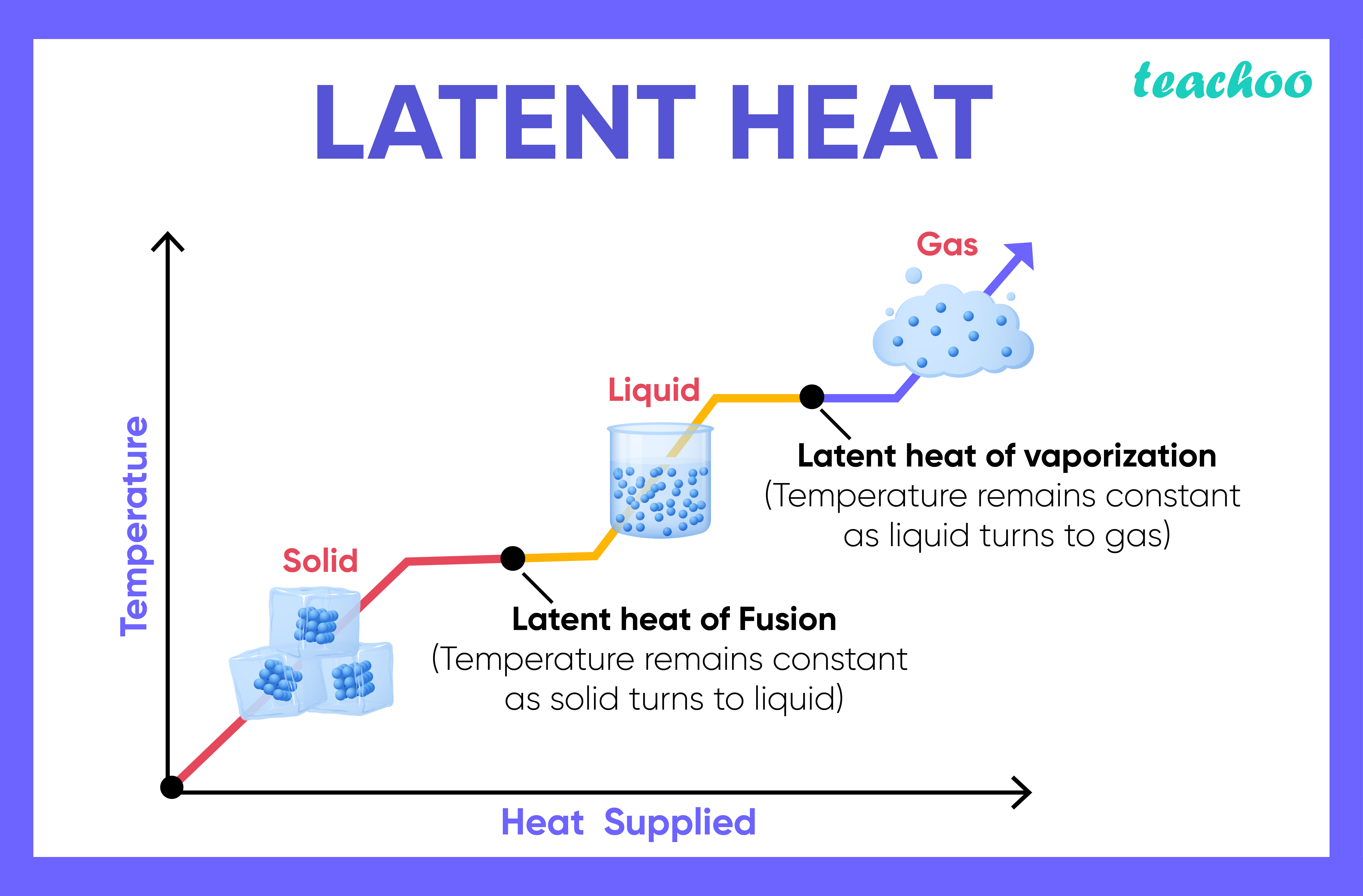
Evaporation Condensation And Heat Transfer . this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. Condensation is the change of state. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,.
from exorpbsao.blob.core.windows.net
Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v.
Heat Lot Definition at Mamie Lyons blog
Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. Condensation is the change of state.
From instrumentationtools.com
HVAC Refrigeration Cycle Evaporation Condensation And Heat Transfer evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. . Evaporation Condensation And Heat Transfer.
From www.tec-science.com
Specific latent heat of condensation tecscience Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. evaporation is the conversion of a liquid to its vapor. Evaporation Condensation And Heat Transfer.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a. Evaporation Condensation And Heat Transfer.
From www.youtube.com
Evaporation and Condensation of Water Drop Simultaneous Heat and Mass Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole. Evaporation Condensation And Heat Transfer.
From www.researchgate.net
Relation between condensation heat transfer coefficient and partial Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. this book includes 25 advanced and revised contributions, and it. Evaporation Condensation And Heat Transfer.
From me-mechanicalengineering.com
Modes of Heat Transfer Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. evaporation is the conversion of a liquid to its. Evaporation Condensation And Heat Transfer.
From www.scitusacademics.com
Evaporation, Condensation and Heat transfer Scitus Academics Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a. Evaporation Condensation And Heat Transfer.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Evaporation Condensation And Heat Transfer evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. Condensation is the change of state.. Evaporation Condensation And Heat Transfer.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation. Evaporation Condensation And Heat Transfer.
From www.researchgate.net
Energy balance diagram of a solar water evaporation system based on Evaporation Condensation And Heat Transfer evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a. Evaporation Condensation And Heat Transfer.
From www.alaquainc.com
Investigating the Heat Transfer Principles of the Evaporator Evaporation Condensation And Heat Transfer Condensation is the change of state. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. evaporation is the conversion of a liquid to its vapor below the. Evaporation Condensation And Heat Transfer.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by. Evaporation Condensation And Heat Transfer.
From learningdbgoldberg.z21.web.core.windows.net
Explain The Three Methods Of Heat Transfer Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the molar heat of vaporization \(\left( \delta h_\text{vap}. Evaporation Condensation And Heat Transfer.
From www.slideserve.com
PPT Ch. 27 Evaporation, Condensation, and Precipitation PowerPoint Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. Condensation is the change of state. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. this book includes 25 advanced and revised contributions, and it. Evaporation Condensation And Heat Transfer.
From www.tec-science.com
Specific latent heat of condensation tecscience Evaporation Condensation And Heat Transfer the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. Condensation is the change of state. this book includes. Evaporation Condensation And Heat Transfer.
From fixlibrarybloesgwyrum.z13.web.core.windows.net
Heat Transfer Circuit Diagram Evaporation Condensation And Heat Transfer evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\]. Evaporation Condensation And Heat Transfer.
From www.youtube.com
Biowork 6p137151 Heat Transfer, Evaporation, Condensation, Stripping Evaporation Condensation And Heat Transfer Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\). Evaporation Condensation And Heat Transfer.
From www.vedantu.com
Evaporation and Condensation Definition, Applications and Difference Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. Condensation is the change of state. this book includes 25 advanced and revised contributions, and it. Evaporation Condensation And Heat Transfer.
From www.chemicalslearning.com
Types of Convection Heat Transfer, Examples and Rate of Heat Transfer Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by. Evaporation Condensation And Heat Transfer.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. Condensation is the change of state. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by. Evaporation Condensation And Heat Transfer.
From www.scribd.com
Evaporation Condensation and Heat Transfer PDF Humidity Relative Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted.. Evaporation Condensation And Heat Transfer.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing Evaporation Condensation And Heat Transfer the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. Condensation is the change of state. evaporation is the conversion of a liquid to its vapor. Evaporation Condensation And Heat Transfer.
From stock.adobe.com
The mechanisms of heat transfer conduction, convection, radiation Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. Condensation is the change of state. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. evaporation is the conversion of a liquid to its vapor. Evaporation Condensation And Heat Transfer.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and. Evaporation Condensation And Heat Transfer.
From www.studyiq.com
Evaporation and Condensation, Definition, Process, Types, Difference Evaporation Condensation And Heat Transfer evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is. Evaporation Condensation And Heat Transfer.
From www.buzzle.com
Conduction, Convection, and Radiation 3 Modes of Heat Transfer Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by. Evaporation Condensation And Heat Transfer.
From www.researchgate.net
Liquid film evaporation and condensation heat transfer inside a Evaporation Condensation And Heat Transfer Condensation is the change of state. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly. Evaporation Condensation And Heat Transfer.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and. Evaporation Condensation And Heat Transfer.
From www.bol.com
Evaporation, Condensation and Heat transfer 9789533075839 Boeken bol Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. Condensation is the change of state. this book includes 25 advanced and revised contributions, and it. Evaporation Condensation And Heat Transfer.
From circuitgonelladrianxm.z22.web.core.windows.net
Show The Phase Change Diagram Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\]. Evaporation Condensation And Heat Transfer.
From mycourses.co.za
The process responsible for the release of heat energy by the earth to Evaporation Condensation And Heat Transfer the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation.. Evaporation Condensation And Heat Transfer.
From exorpbsao.blob.core.windows.net
Heat Lot Definition at Mamie Lyons blog Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the. Evaporation Condensation And Heat Transfer.
From www.pnas.org
Mass and heat transfer between evaporation and condensation surfaces Evaporation Condensation And Heat Transfer the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the. Evaporation Condensation And Heat Transfer.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet Evaporation Condensation And Heat Transfer the heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted. evaporation is the conversion of a liquid to its. Evaporation Condensation And Heat Transfer.
From www.youtube.com
Evaporation Heat transfer coeff (DRAFT video) YouTube Evaporation Condensation And Heat Transfer this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling, (2) condensation. this book includes 25 advanced and revised contributions, and it covers mainly (1) evaporation and boiling,. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) is the heat absorbed by one mole of a substance as it is converted.. Evaporation Condensation And Heat Transfer.