Titration Curve Strong Base . We'll take hydrochloric acid and sodium hydroxide as typical of a strong. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. titration of a weak acid with a strong base. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. Shapes of curves and some definitions. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. Titration of strong acids and strong bases. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. titration curves for strong acid v strong base. In a titration of a strong. The ph curve diagram below represents the titration of a strong acid.
from general.chemistrysteps.com
titration of a weak acid with a strong base. The ph curve diagram below represents the titration of a strong acid. Titration of strong acids and strong bases. Shapes of curves and some definitions. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. titration curves for strong acid v strong base.
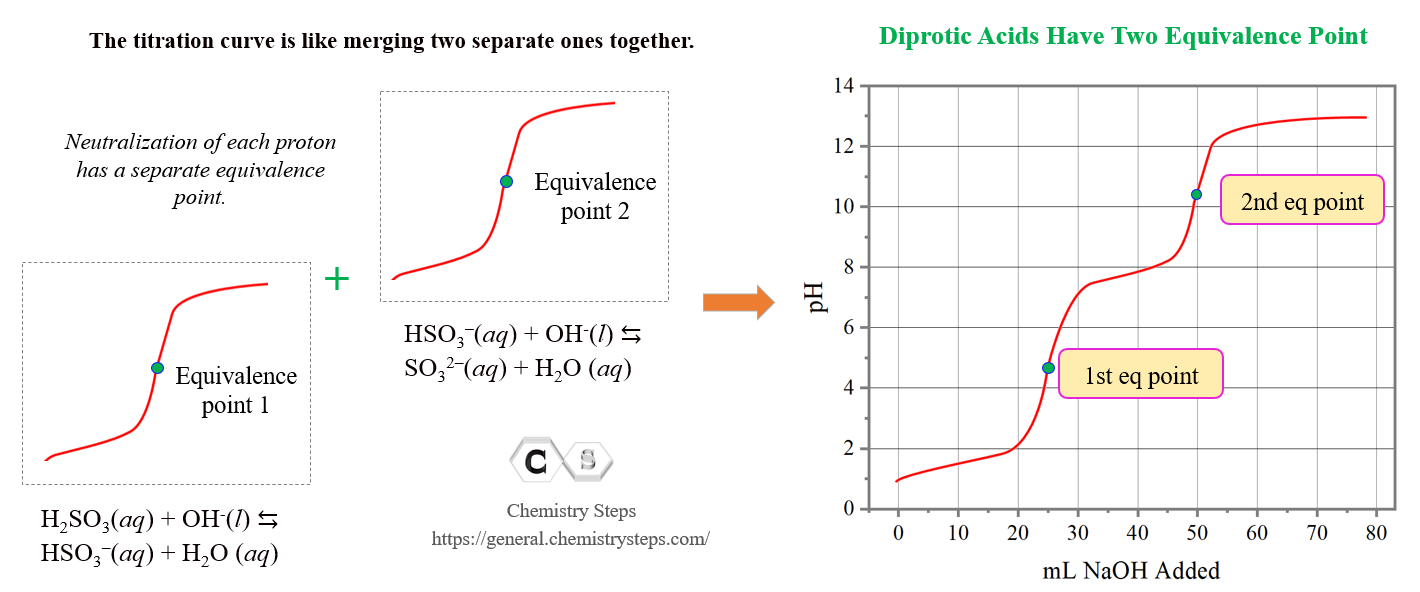
Titration of a Polyprotic Acids Chemistry Steps
Titration Curve Strong Base Titration of strong acids and strong bases. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. titration of a weak acid with a strong base. In a titration of a strong. titration curves for strong acid v strong base. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. Shapes of curves and some definitions. Titration of strong acids and strong bases. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. The ph curve diagram below represents the titration of a strong acid. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Strong Base titration of a weak acid with a strong base. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. Titration of strong acids and strong bases. The ph curve diagram below represents the titration of a strong acid. The titration curve shown in figure 1. Titration Curve Strong Base.
From www.numerade.com
SOLVEDDraw the general titration curve for a strong acid titrated with Titration Curve Strong Base Shapes of curves and some definitions. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. In a titration of a strong. The ph curve diagram below represents. Titration Curve Strong Base.
From www.numerade.com
SOLVED What kind of titration is shown in the graph below? PH Titrant Titration Curve Strong Base titration of a weak acid with a strong base. In a titration of a strong. Shapes of curves and some definitions. titration curves for strong acid v strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Imagine we are titrating a. Titration Curve Strong Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Strong Base Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. In a titration of a strong. We'll take hydrochloric acid and sodium hydroxide as typical of a strong.. Titration Curve Strong Base.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Strong Base titration curves for strong acid v strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. We'll take hydrochloric acid and sodium hydroxide as typical. Titration Curve Strong Base.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Curve Strong Base Shapes of curves and some definitions. titration of a weak acid with a strong base. The ph curve diagram below represents the titration of a strong acid. In a titration of a strong. titration curves for strong acid v strong base. Titration of strong acids and strong bases. The titration curve is a graph of the volume of. Titration Curve Strong Base.
From exolxuobk.blob.core.windows.net
How To Do Strong Acid Strong Base Titration at Cole blog Titration Curve Strong Base titration curves for strong acid v strong base. titration of a weak acid with a strong base. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m. Titration Curve Strong Base.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Strong Base Titration of strong acids and strong bases. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. The ph curve diagram below represents. Titration Curve Strong Base.
From mavink.com
Strong Acid And Strong Base Titration Curve Titration Curve Strong Base In a titration of a strong. titration of a weak acid with a strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. The ph curve diagram below represents the titration of a strong acid. Shapes of curves and some definitions. The titration curve is a graph of the volume of titrant, or in our. Titration Curve Strong Base.
From www.researchgate.net
2 Curve for the titration of a strong base with a strong acid Titration Curve Strong Base titration curves for strong acid v strong base. In a titration of a strong. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. titration of a weak acid with a strong base. Titration of strong acids and. Titration Curve Strong Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Strong Base titration of a weak acid with a strong base. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Shapes of curves and some definitions. The titration curve shown in figure 1 is for the titration of 25.00 ml of. Titration Curve Strong Base.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Strong Base titration curves for strong acid v strong base. titration of a weak acid with a strong base. In a titration of a strong. The ph curve diagram below represents the titration of a strong acid. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Titration of strong acids and strong bases. When titrating a strong. Titration Curve Strong Base.
From www.researchgate.net
Schematic plot of titration curves of strong acid with strong base Titration Curve Strong Base Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The ph curve diagram below represents the titration of a strong acid. titration curves for strong acid v strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100. Titration Curve Strong Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Strong Base Titration of strong acids and strong bases. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. In a titration of a strong. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. The ph curve diagram below represents the titration of a strong acid. titration curves for strong acid. Titration Curve Strong Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Strong Base Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. In a titration of a strong. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Imagine we are titrating a strong acid such as hydrochloric acid against. Titration Curve Strong Base.
From mungfali.com
Acid Base Titration Graph Titration Curve Strong Base titration curves for strong acid v strong base. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base. Titration Curve Strong Base.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration Curve Strong Base The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. titration curves for strong acid v strong base. The ph curve diagram below represents the titration of a strong acid. Titration of strong acids and strong bases. We'll take hydrochloric acid and sodium hydroxide as. Titration Curve Strong Base.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Strong Base The ph curve diagram below represents the titration of a strong acid. titration curves for strong acid v strong base. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. In a titration of a strong. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100. Titration Curve Strong Base.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. In a titration of a strong. titration curves for strong acid. Titration Curve Strong Base.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Strong Base Shapes of curves and some definitions. In a titration of a strong. titration curves for strong acid v strong base. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. The ph curve diagram below represents the titration of. Titration Curve Strong Base.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Titration Curve Strong Base Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The ph curve diagram below represents the titration of a strong acid. When titrating a strong acid, such as. Titration Curve Strong Base.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Titration Curve Strong Base The ph curve diagram below represents the titration of a strong acid. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide,. Titration Curve Strong Base.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve Strong Base In a titration of a strong. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. titration curves for strong acid v strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. titration of. Titration Curve Strong Base.
From www.slideserve.com
PPT Buffers and Acid/Base Titration PowerPoint Presentation, free Titration Curve Strong Base titration curves for strong acid v strong base. Titration of strong acids and strong bases. In a titration of a strong. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Imagine we are titrating a strong acid such. Titration Curve Strong Base.
From mungfali.com
Titration Curve Labeled Titration Curve Strong Base When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. Shapes of curves and some definitions. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Titration of strong acids and strong bases. The ph curve diagram below represents the titration of a strong acid. titration curves for strong. Titration Curve Strong Base.
From www.vrogue.co
Strong Acid And Strong Base Titration Curve vrogue.co Titration Curve Strong Base The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Titration of strong acids and strong bases. titration curves for strong acid v strong base. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The titration curve. Titration Curve Strong Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Curve Strong Base The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The. Titration Curve Strong Base.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. In a titration of a strong. Titration of strong acids and strong bases. Shapes of curves and some definitions. The ph curve diagram below represents the titration of a strong acid. The titration curve is a graph of the volume of titrant, or in our case the volume. Titration Curve Strong Base.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Curve Strong Base In a titration of a strong. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. titration curves for strong acid v strong base. The ph curve diagram below represents the titration of a strong acid. titration of a weak acid with a strong. Titration Curve Strong Base.
From philschatz.com
AcidBase Titrations · Chemistry Titration Curve Strong Base The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. titration curves for strong acid v strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Titration of strong. Titration Curve Strong Base.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Strong Base The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. In a titration of a strong. The ph curve diagram below represents. Titration Curve Strong Base.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Strong Base In a titration of a strong. The ph curve diagram below represents the titration of a strong acid. titration of a weak acid with a strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Titration of strong acids and strong bases. Imagine we are titrating a strong acid such as hydrochloric acid against a. Titration Curve Strong Base.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Strong Base Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. The ph curve diagram below represents the titration of a strong acid. titration of a weak acid with a strong base. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly. In a titration. Titration Curve Strong Base.
From www.chegg.com
Solved The titration curve produced when a strong base is Titration Curve Strong Base titration of a weak acid with a strong base. Shapes of curves and some definitions. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Titration of strong acids and strong bases. Imagine we are titrating a strong acid such as hydrochloric acid against a. Titration Curve Strong Base.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Curve Strong Base Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as. titration of a weak acid with a strong base. The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the ph. In a titration of a strong. The ph curve. Titration Curve Strong Base.