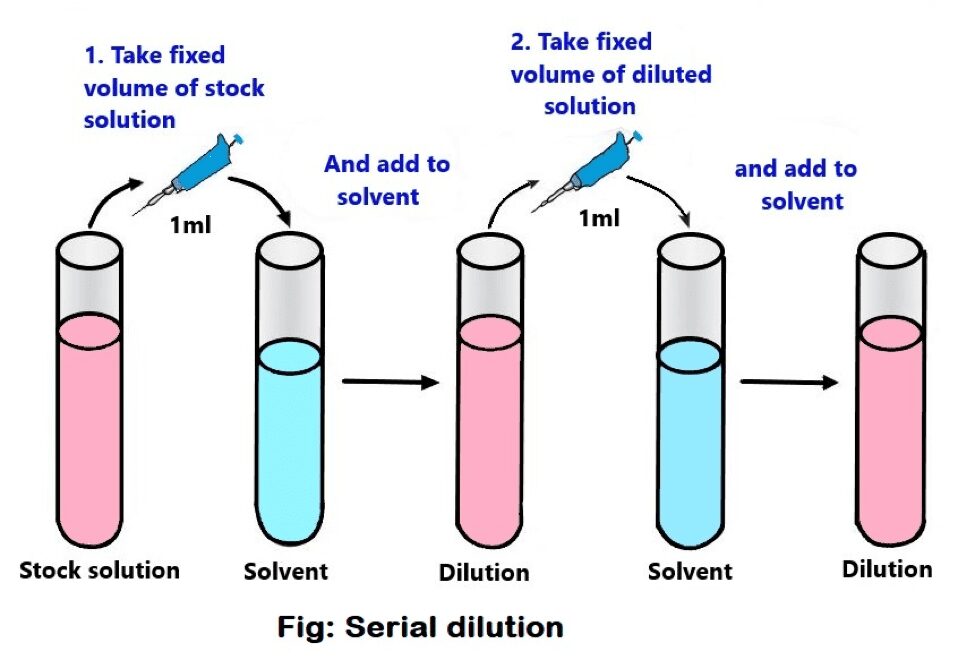
How Well Does Dilution Work . Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the addition of solvent,. Often, a worker will need to change the concentration of a solution by changing the. A dilute solution is one. When additional water is added to an aqueous solution, the concentration of that solution decreases. Learn how to dilute and concentrate solutions. This is because the amount of solute remains the same, while the volume of the. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water.
from sciencequery.com
Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent,. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. We will begin our discussion of solution concentration with two related and relative terms: Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. A dilute solution is one. This is because the amount of solute remains the same, while the volume of the. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. When additional water is added to an aqueous solution, the concentration of that solution decreases.
What is serial dilution method? And how to calculate? Science Query
How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the addition of solvent,. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. Learn how to dilute and concentrate solutions. When additional water is added to an aqueous solution, the concentration of that solution decreases. We will begin our discussion of solution concentration with two related and relative terms: This is because the amount of solute remains the same, while the volume of the.
From dxoecpgvz.blob.core.windows.net
How To Dilutions at Christopher Stevenson blog How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: This is because. How Well Does Dilution Work.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 How Well Does Dilution Work A dilute solution is one. This is because the amount of solute remains the same, while the volume of the. Dilution is the addition of solvent,. When additional water is added to an aqueous solution, the concentration of that solution decreases. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various. How Well Does Dilution Work.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How Well Does Dilution Work Dilution is the addition of solvent,. Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. Concentration is the removal of. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. A dilute solution is one. Dilution is the addition of. How Well Does Dilution Work.
From klaujfijv.blob.core.windows.net
How Do Dilutions Work at Michael Stanley blog How Well Does Dilution Work Dilution is the addition of solvent,. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. This is because the amount of solute remains the same, while the volume of the. Dilution is. How Well Does Dilution Work.
From klaujfijv.blob.core.windows.net
How Do Dilutions Work at Michael Stanley blog How Well Does Dilution Work Dilution is the addition of solvent,. Often, a worker will need to change the concentration of a solution by changing the. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as. How Well Does Dilution Work.
From www.infopathy.com
DNA Transduction Induced by Weak EM Field Infopathy How Well Does Dilution Work A dilute solution is one. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This is because the amount of solute remains the same, while the volume of the. When additional water is added to an aqueous solution, the concentration of that solution decreases. Solutions can be prepared by diluting a more concentrated. How Well Does Dilution Work.
From centerpointsecurities.com
Stock Dilution How it Works and What to Be Aware Of How Well Does Dilution Work A dilute solution is one. This is because the amount of solute remains the same, while the volume of the. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent,. Often, a worker will need to change the concentration of. How Well Does Dilution Work.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free How Well Does Dilution Work We will begin our discussion of solution concentration with two related and relative terms: This is because the amount of solute remains the same, while the volume of the. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent,. When. How Well Does Dilution Work.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution, the concentration of that solution decreases. This is because the amount of solute remains the same, while the volume of the. We will begin our discussion of solution concentration with two related and relative terms:. How Well Does Dilution Work.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. We will begin our discussion of solution concentration with two related and relative terms: Learn how to dilute and concentrate solutions. A dilute solution is one. Concentration is the removal of. When additional water is added to an aqueous solution, the concentration of that solution. How Well Does Dilution Work.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the addition of solvent,. When additional water is added to an aqueous solution, the concentration of that solution. How Well Does Dilution Work.
From www.youtube.com
Well that should stop my dilutions 👍😭 YouTube How Well Does Dilution Work Concentration is the removal of. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the. When additional water is added to an aqueous solution, the concentration of that solution decreases. Dilution is the addition of solvent,. A dilute. How Well Does Dilution Work.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. This is because the amount of solute remains the same, while the volume of the. Dilution is the addition of solvent,. Concentration is the removal of. A dilute solution is one. Dilution is the addition of solvent,. How Well Does Dilution Work.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent,. Solutions can be prepared by diluting a more concentrated solution. How Well Does Dilution Work.
From ceysjldb.blob.core.windows.net
How To Graph Serial Dilutions at Linda Pike blog How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. How Well Does Dilution Work.
From www.mostlymetrics.com
How does dilution work? by CJ Gustafson Mostly metrics How Well Does Dilution Work Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the addition of solvent,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution, the concentration of that solution decreases. This is because the amount of. How Well Does Dilution Work.
From www.slideshare.net
How to calculate dilution Step How Well Does Dilution Work Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Concentration is the removal of. Often, a worker will need to change the concentration of a solution by changing the. When additional water is added to an aqueous solution, the concentration of that solution decreases. A dilute solution is one. Learn how to. How Well Does Dilution Work.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. This is because the amount of solute remains the same, while the volume of the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Concentration is the removal of. When additional water is. How Well Does Dilution Work.
From loegnwkyw.blob.core.windows.net
Serial Dilution Equation Examples at Keith Rush blog How Well Does Dilution Work We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Dilution is. How Well Does Dilution Work.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. When additional water is added to an aqueous solution, the concentration of that solution decreases. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. We will. How Well Does Dilution Work.
From klaujfijv.blob.core.windows.net
How Do Dilutions Work at Michael Stanley blog How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent,. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Dilution is the act of lowering the. How Well Does Dilution Work.
From aliceblueonline.com
What Is Share Dilution? How Does Dilution Work? How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. A dilute solution is one. Dilution is the addition of solvent,. Concentration. How Well Does Dilution Work.
From alejandrocremades.com
How Does Dilution Work In Startups? How Well Does Dilution Work Concentration is the removal of. This is because the amount of solute remains the same, while the volume of the. When additional water is added to an aqueous solution, the concentration of that solution decreases. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. We will. How Well Does Dilution Work.
From www.slideshare.net
Serial dilution How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. This is because the amount of solute remains the same, while the volume of the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution,. How Well Does Dilution Work.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube How Well Does Dilution Work This is because the amount of solute remains the same, while the volume of the. When additional water is added to an aqueous solution, the concentration of that solution decreases. Learn how to dilute and concentrate solutions. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water.. How Well Does Dilution Work.
From www.youtube.com
Dilutions Explained with Problems YouTube How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. When additional water is added to an aqueous solution, the concentration of that solution decreases. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Concentration is the removal of.. How Well Does Dilution Work.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Dilution is the addition of solvent,. When additional water is added to an aqueous solution, the concentration of that solution decreases. This is because the amount of solute remains the same, while the volume of the. A. How Well Does Dilution Work.
From www.youtube.com
How do Dilution Ratios work? (For Detailing Product Chemicals) YouTube How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. We will begin our discussion of. How Well Does Dilution Work.
From medium.com
If symbolic, please include that as well. The Rapture of our Collective How Well Does Dilution Work Dilution is the addition of solvent,. Concentration is the removal of. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. We will begin our discussion of solution concentration with two related and relative terms: This is because the amount of solute remains the same, while the volume of the. When additional water. How Well Does Dilution Work.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis How Well Does Dilution Work Concentration is the removal of. Dilution is the addition of solvent,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution, the concentration of that solution decreases. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. We. How Well Does Dilution Work.
From www.youtube.com
AS Biology How to calculate serial and simple dilutions YouTube How Well Does Dilution Work Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as. How Well Does Dilution Work.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How Well Does Dilution Work This is because the amount of solute remains the same, while the volume of the. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. When additional water is added to an aqueous solution, the concentration of that solution decreases.. How Well Does Dilution Work.
From ar.inspiredpencil.com
Dilute Science How Well Does Dilution Work Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents, such as water. Dilution is the addition of solvent,. Learn how to dilute and concentrate solutions. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. We will begin our discussion of solution. How Well Does Dilution Work.
From www.researchgate.net
Schematic illustration of predilution and postdilution online How Well Does Dilution Work A dilute solution is one. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Dilution is the addition of solvent,. Learn how to dilute and concentrate solutions. This is because the amount of solute remains the same, while the volume of the. We will begin our discussion of solution concentration with two. How Well Does Dilution Work.
From mmerevise.co.uk
Concentrations and Dilutions MME How Well Does Dilution Work Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Solutions can be prepared by diluting a more concentrated solution. How Well Does Dilution Work.