Laboratory Equipment Validation Sop . Requirements to perform a validation or verification study. This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. In writing of the sop take. Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and.
from www.chemwifi.com
In writing of the sop take. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Requirements to perform a validation or verification study. This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. Note that validation of laboratory information systems (lis) must also be counted under equipment validation.
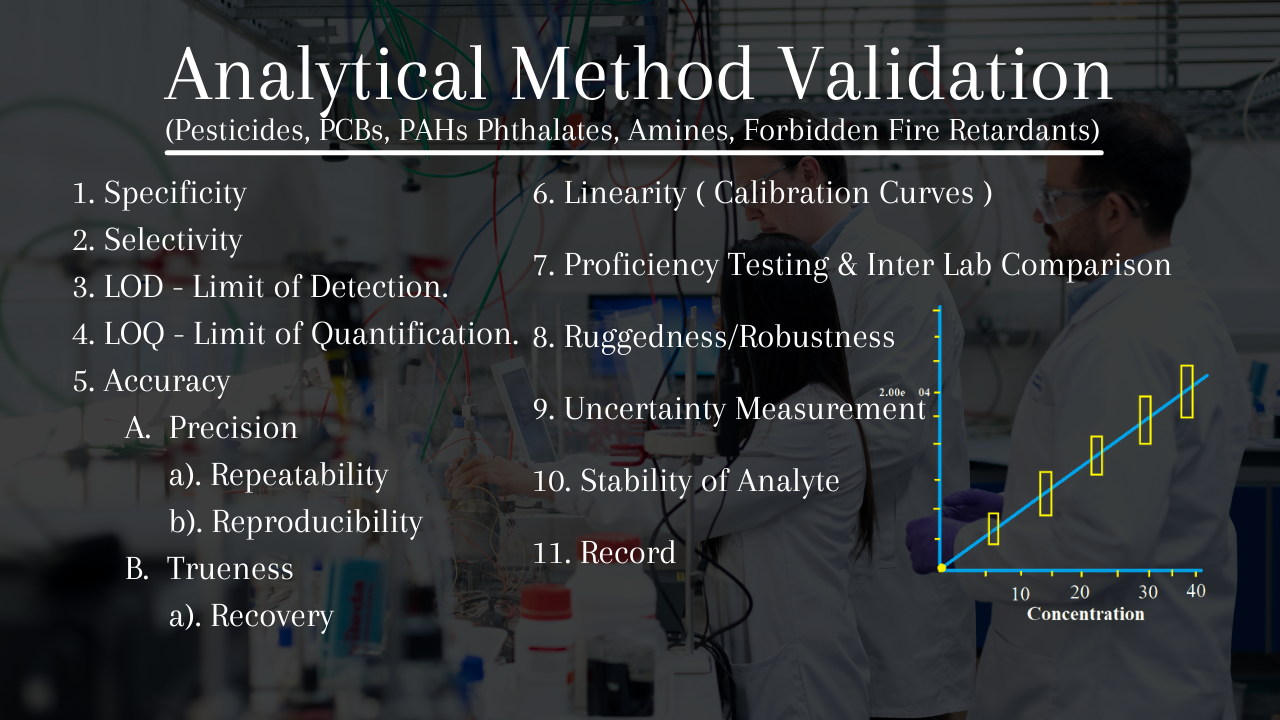
Analytical Method validation for Pesticides, PCBs, PAHs Phthalates
Laboratory Equipment Validation Sop The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. Requirements to perform a validation or verification study. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. In writing of the sop take. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Note that validation of laboratory information systems (lis) must also be counted under equipment validation.
From www.scribd.com
Laboratory Management SOP Sample Personal Protective Equipment Laboratory Equipment Validation Sop Requirements to perform a validation or verification study. Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a. Laboratory Equipment Validation Sop.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Laboratory Equipment Validation Sop Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. Requirements to perform a validation or verification study. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be. Laboratory Equipment Validation Sop.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Laboratory Equipment Validation Sop In writing of the sop take. Requirements to perform a validation or verification study. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Note. Laboratory Equipment Validation Sop.
From dxokdbkuu.blob.core.windows.net
Laboratory Sample Receiving Sop at Christina Biggins blog Laboratory Equipment Validation Sop Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Requirements to perform a validation or verification study. The metrology laboratory follows this procedure to ensure that all laboratory methods selected,. Laboratory Equipment Validation Sop.
From www.scribd.com
Validation Criteria Checklist Verification And Validation Laboratory Equipment Validation Sop The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. In writing of the sop take. The purpose of this toolkit is to assist laboratories. Laboratory Equipment Validation Sop.
From issuu.com
Vt tmp1200 10 validation plan equipment qualification template r02 by Laboratory Equipment Validation Sop The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The purpose of this toolkit is to assist laboratories in determining the difference between a. Laboratory Equipment Validation Sop.
From www.sampletemplate.my.id
Validation Certificate Template Sampletemplate.my.id Laboratory Equipment Validation Sop The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs. Laboratory Equipment Validation Sop.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Laboratory Equipment Validation Sop In writing of the sop take. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and. Laboratory Equipment Validation Sop.
From www.presentationeze.com
Stages to equipment validation PresentationEZE Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be. Laboratory Equipment Validation Sop.
From www.chemwifi.com
Analytical Method validation for Pesticides, PCBs, PAHs Phthalates Laboratory Equipment Validation Sop Note that validation of laboratory information systems (lis) must also be counted under equipment validation. Requirements to perform a validation or verification study. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. In writing of the sop take. The purpose of the sop is to. Laboratory Equipment Validation Sop.
From info.mesalabs.com
Pharmaceutical Aseptic Manufacturing Sterilization Validation Mesa Labs Laboratory Equipment Validation Sop The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed. Laboratory Equipment Validation Sop.
From venngage.com
Standard Operating Procedure for Laboratory Tests Template Venngage Laboratory Equipment Validation Sop The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. Requirements to perform a validation or verification study. The aim of this validation guideline is to provide a. Laboratory Equipment Validation Sop.
From dxokdbkuu.blob.core.windows.net
Laboratory Sample Receiving Sop at Christina Biggins blog Laboratory Equipment Validation Sop Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated. Laboratory Equipment Validation Sop.
From pharmaguddu.com
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu Laboratory Equipment Validation Sop The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be. Laboratory Equipment Validation Sop.
From fens.sabanciuniv.edu
Standard Operating Procedures (SOPs) FACULTY OF ENGINEERING AND Laboratory Equipment Validation Sop The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. Requirements to perform a validation or verification study. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The aim of this validation guideline is to provide a clear statement of the scope,. Laboratory Equipment Validation Sop.
From exyinuwis.blob.core.windows.net
What Does Sop Mean In Health And Safety at Troy Stallard blog Laboratory Equipment Validation Sop The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The aim of this validation guideline is to provide a clear statement of the scope,. Laboratory Equipment Validation Sop.
From biotechserv601227982.wordpress.com
The Importance of Lab Equipment Validation Biotechnical Services Laboratory Equipment Validation Sop Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. In writing of. Laboratory Equipment Validation Sop.
From pubhtml5.com
SOPQA07 REV.17 EQUIPMENT CALIBRATION AND LABORATORY CONTROL Nukanya Laboratory Equipment Validation Sop In writing of the sop take. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The metrology laboratory follows this procedure to ensure that. Laboratory Equipment Validation Sop.
From www.sampletemplates.com
10+ Validation Plan Templates Sample Templates Laboratory Equipment Validation Sop Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. This procedure specifies the schedule and. Laboratory Equipment Validation Sop.
From www.biotechserv.com
Lab Equipment for Regulated Industries Qualification and Validation Laboratory Equipment Validation Sop The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. In writing of the sop take. Analytical equipment qualification is the collection of. Laboratory Equipment Validation Sop.
From pharmaceuticalcarrier.com
Standard Operating Procedure (SOP) for Microscope Pharma Career Laboratory Equipment Validation Sop In writing of the sop take. Note that validation of laboratory information systems (lis) must also be counted under equipment validation. This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of this toolkit. Laboratory Equipment Validation Sop.
From www.scribd.com
Cleaning Validation SOP PDF Verification And Validation Dose Laboratory Equipment Validation Sop Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. In writing of the sop take. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The purpose of this toolkit is to assist laboratories in determining the difference between a. Laboratory Equipment Validation Sop.
From www.scribd.com
Cleaning Validation Master Plan Verification And Validation Laboratory Equipment Validation Sop Note that validation of laboratory information systems (lis) must also be counted under equipment validation. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. This procedure specifies. Laboratory Equipment Validation Sop.
From www.biotechserv.com
Lab Equipment Validation Technicians Who are They and What do They Do? Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. In writing of the sop take. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. Note. Laboratory Equipment Validation Sop.
From www.biotechserv.com
Best Practices for Lab Equipment Validation Laboratory Equipment Validation Sop Requirements to perform a validation or verification study. In writing of the sop take. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. This procedure specifies. Laboratory Equipment Validation Sop.
From www.slideshare.net
SOP Water Analysis Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of. Laboratory Equipment Validation Sop.
From old.sermitsiaq.ag
Equipment Sop Template Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The aim of this validation. Laboratory Equipment Validation Sop.
From www.scribd.com
SOP Equipment Validation Verification And Validation Specification Laboratory Equipment Validation Sop Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. This procedure. Laboratory Equipment Validation Sop.
From www.advipro.com
Equipment validation Advipro Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. In writing. Laboratory Equipment Validation Sop.
From www.mybiosource.com
Best Practices for Maintaining CLIA Certification and Compliance Laboratory Equipment Validation Sop The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Analytical equipment qualification is the collection of documented evidence, which demonstrates that specific equipment performs suitably for its. In writing of the sop take. The purpose of this toolkit is to assist laboratories in determining the difference between a. Laboratory Equipment Validation Sop.
From www.slideserve.com
PPT Lab Equipment Validation Services in San Diego PowerPoint Laboratory Equipment Validation Sop The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. Requirements to perform a validation or verification study. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The aim of this validation guideline is. Laboratory Equipment Validation Sop.
From www.pinterest.com
SOP for Internal and External Calibration of Instrument Standard Laboratory Equipment Validation Sop In writing of the sop take. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The aim of this validation guideline is to provide. Laboratory Equipment Validation Sop.
From www.coolvacuum.com
Lab Equipment Validation & Support by Coolvacuum Laboratory Equipment Validation Sop This procedure specifies the schedule and requirements for maintenance, performance, calibration, and verification of laboratory testing. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The aim of this validation. Laboratory Equipment Validation Sop.
From www.allbusinesstemplates.com
Process Validation Templates at Laboratory Equipment Validation Sop The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and. Note that validation of laboratory information systems (lis) must also be counted under equipment validation. The purpose of the sop is to describe validation practices for laboratory instrument/equipment to be validated or calibrated and the confirmatory. The aim of this validation. Laboratory Equipment Validation Sop.
From drug-dev.com
STANDARD OPERATING PROCEDURES How Writing an Effective SOP Can Laboratory Equipment Validation Sop The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. In writing of the sop take. The purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification, when each should be performed,. The metrology laboratory follows. Laboratory Equipment Validation Sop.