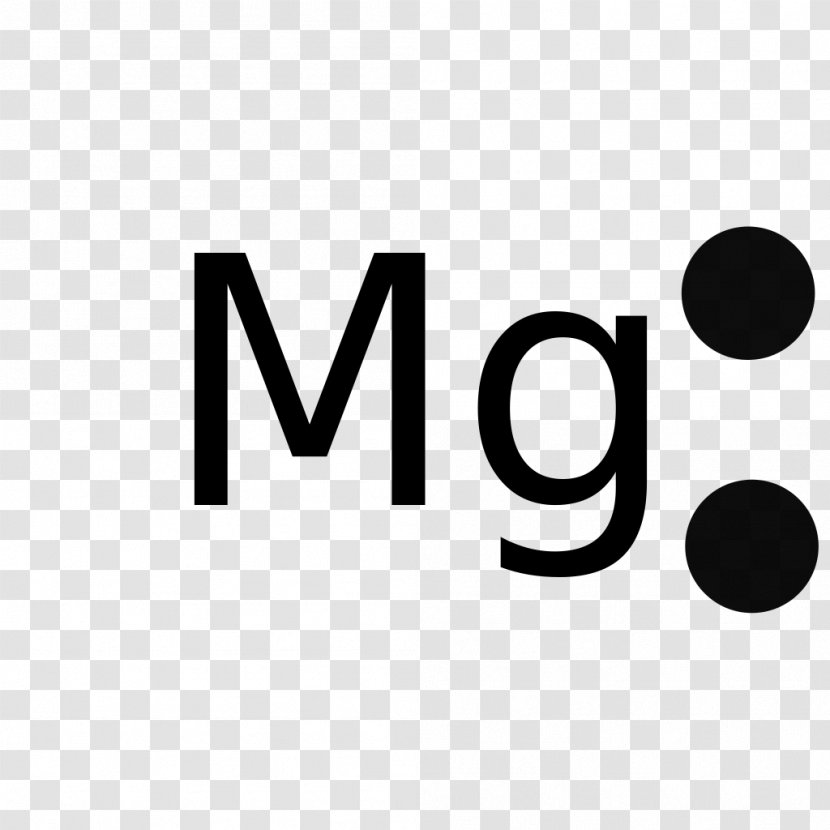Chlorine Valence Electrons In Magnesium . The highest principal quantum number is 2. Magnesium has two valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. Calcium would have two valence electrons, since it is in group iia (group 2). The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Elements in any one group (or column) have the same number of valence electrons; Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2.
from pnghut.com
Elements in any one group (or column) have the same number of valence electrons; The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Chlorine is in group viia (group 17), so it would have seven valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Magnesium has two valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The highest principal quantum number is 2. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Calcium would have two valence electrons, since it is in group iia (group 2). But for most of the transition.
Lewis Structure Magnesium Chloride Electron Diagram Rectangle Dot Transparent PNG
Chlorine Valence Electrons In Magnesium Chlorine is in group viia (group 17), so it would have seven valence electrons. Magnesium has two valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. But for most of the transition. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The highest principal quantum number is 2. Elements in any one group (or column) have the same number of valence electrons; Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. The highest principal quantum number is 2. Elements in any one group (or column) have the same number of valence electrons; Magnesium has two valence electrons. 93 rows you may assume the. Chlorine Valence Electrons In Magnesium.
From www.youtube.com
Valency of magnesium CBSE Class 9 Chemistry notes YouTube Chlorine Valence Electrons In Magnesium The highest principal quantum number is 2. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group. Chlorine Valence Electrons In Magnesium.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Chlorine Valence Electrons In Magnesium 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Elements in any one group (or column) have the same number of valence electrons; Chlorine is in group viia (group. Chlorine Valence Electrons In Magnesium.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Valence Electrons In Magnesium The highest principal quantum number is 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine is in group viia (group 17), so it would have seven valence electrons. Elements. Chlorine Valence Electrons In Magnesium.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Chlorine Valence Electrons In Magnesium Magnesium has two valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. The highest principal quantum number is 2. But for most of. Chlorine Valence Electrons In Magnesium.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Chlorine Valence Electrons In Magnesium But for most of the transition. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Elements in any one group (or column) have the same number of valence electrons; Calcium would have two valence electrons, since it is in group iia (group 2). The alkali metals lithium and sodium each have only one valence electron, the alkaline. Chlorine Valence Electrons In Magnesium.
From schematicellsclours.z21.web.core.windows.net
Magnesium Atomic Structure Diagram Chlorine Valence Electrons In Magnesium Calcium would have two valence electrons, since it is in group iia (group 2). Elements in any one group (or column) have the same number of valence electrons; The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. The alkali metals lithium. Chlorine Valence Electrons In Magnesium.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine Valence Electrons In Magnesium The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons. Chlorine Valence Electrons In Magnesium.
From utedzz.blogspot.com
Periodic Table Electron Configuration Orbital Diagram Periodic Table Timeline Chlorine Valence Electrons In Magnesium Magnesium has two valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. 93 rows you may assume the valences of the chemical elements—the number of. Chlorine Valence Electrons In Magnesium.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Elements in any one group (or column) have the same number of valence electrons; The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Calcium would. Chlorine Valence Electrons In Magnesium.
From slideplayer.com
Understanding Chemical Formula ppt download Chlorine Valence Electrons In Magnesium The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. The highest principal quantum number is 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Elements in any one group (or column) have the same number of valence. Chlorine Valence Electrons In Magnesium.
From slideplayer.com
How to Draw Lewis Structures ppt download Chlorine Valence Electrons In Magnesium But for most of the transition. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Magnesium has two valence electrons. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Calcium would have two valence. Chlorine Valence Electrons In Magnesium.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Chlorine Valence Electrons In Magnesium Magnesium has two valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have two valence electrons, since it is in group iia (group 2). Carbon’s. Chlorine Valence Electrons In Magnesium.
From slideplayer.com
Ionic vs. Molecular Compounds ppt download Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Chlorine is in group viia (group 17), so it would have seven valence electrons. The highest principal quantum number is. Chlorine Valence Electrons In Magnesium.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chlorine Valence Electrons In Magnesium Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The highest principal quantum number is 2. The electron arrangement also shows the number of valence electrons which is two for magnesium because there. Chlorine Valence Electrons In Magnesium.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium YouTube Chlorine Valence Electrons In Magnesium Elements in any one group (or column) have the same number of valence electrons; Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Calcium would have two valence electrons, since it is in group iia (group 2). Magnesium has two valence electrons. But for most of the transition. The highest principal quantum number is 2. Chlorine is. Chlorine Valence Electrons In Magnesium.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Chlorine Valence Electrons In Magnesium Calcium would have two valence electrons, since it is in group iia (group 2). Elements in any one group (or column) have the same number of valence electrons; The highest principal quantum number is 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The. Chlorine Valence Electrons In Magnesium.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Chlorine is in group viia (group 17), so it would have seven valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. 93 rows. Chlorine Valence Electrons In Magnesium.
From schematron.org
Dot Diagram Of Magnesium Chloride Wiring Diagram Pictures Chlorine Valence Electrons In Magnesium Chlorine is in group viia (group 17), so it would have seven valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Elements in. Chlorine Valence Electrons In Magnesium.
From www.toppr.com
Give orbital diagram of the followingmagnesium chloride, Chlorine Valence Electrons In Magnesium Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the. Chlorine Valence Electrons In Magnesium.
From favpng.com
Lewis Structure Magnesium Chloride Diagram Electron, PNG, 1050x641px, Lewis Structure, Black And Chlorine Valence Electrons In Magnesium 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Magnesium has two valence electrons. Chlorine is in. Chlorine Valence Electrons In Magnesium.
From galvinconanstuart.blogspot.com
Dot Diagram Of Magnesium Chloride General Wiring Diagram Chlorine Valence Electrons In Magnesium Chlorine is in group viia (group 17), so it would have seven valence electrons. The highest principal quantum number is 2. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. Elements in any one group (or column) have the same number of valence electrons; Carbon’s ground state electron configuration is 1s 2 2s. Chlorine Valence Electrons In Magnesium.
From pnghut.com
Lewis Structure Chlorine Atom Chemistry Chloride Magnesium Transparent PNG Chlorine Valence Electrons In Magnesium The highest principal quantum number is 2. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The alkali metals lithium and sodium each have only one valence electron, the. Chlorine Valence Electrons In Magnesium.
From www.numerade.com
SOLVED Use the electron dot structures to determine chemical formulas for the ionic compounds Chlorine Valence Electrons In Magnesium But for most of the transition. Chlorine is in group viia (group 17), so it would have seven valence electrons. The highest principal quantum number is 2. Elements in any one group (or column) have the same number of valence electrons; The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. 93 rows you. Chlorine Valence Electrons In Magnesium.
From www.toppr.com
Draw electron dot representation for the formation of magnesium chloride. Chlorine Valence Electrons In Magnesium Magnesium has two valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The highest principal quantum number is 2. Carbon’s ground. Chlorine Valence Electrons In Magnesium.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Valence Electrons In Magnesium Calcium would have two valence electrons, since it is in group iia (group 2). Magnesium has two valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Chlorine is in group viia (group 17), so it would have seven valence electrons. The highest principal quantum number is 2. 93 rows you may assume the valences of. Chlorine Valence Electrons In Magnesium.
From pnghut.com
Lewis Structure Magnesium Chloride Electron Diagram Rectangle Dot Transparent PNG Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Chlorine is in group viia (group 17), so it would have seven valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The alkali metals lithium and sodium. Chlorine Valence Electrons In Magnesium.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Valence Electrons In Magnesium Elements in any one group (or column) have the same number of valence electrons; Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The alkali metals lithium and sodium each have only one. Chlorine Valence Electrons In Magnesium.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID651377 Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Calcium would have two valence electrons, since it is in group iia (group 2). Magnesium has two valence electrons. But for most of the transition. Elements in any one group (or column). Chlorine Valence Electrons In Magnesium.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Valence Electrons In Magnesium Magnesium has two valence electrons. But for most of the transition. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Calcium would have two valence electrons, since it is in group iia (group 2). Elements in any one group (or column). Chlorine Valence Electrons In Magnesium.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Chlorine Valence Electrons In Magnesium The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine Valence Electrons In Magnesium.
From www.benjamin-mills.com
Electron configurations Chlorine Valence Electrons In Magnesium The highest principal quantum number is 2. Calcium would have two valence electrons, since it is in group iia (group 2). Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of. Chlorine Valence Electrons In Magnesium.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Chlorine Valence Electrons In Magnesium Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Magnesium has two valence electrons. The alkali metals lithium and sodium each have only one valence electron, the alkaline earth. Chlorine Valence Electrons In Magnesium.
From tutorsuhu.com
What Is The Molecular Structure Of Magnesium Chloride Tutor Suhu Chlorine Valence Electrons In Magnesium Elements in any one group (or column) have the same number of valence electrons; The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two. Chlorine Valence Electrons In Magnesium.
From guidelibstoitering.z21.web.core.windows.net
Magnesium Electron Dot Diagram Chlorine Valence Electrons In Magnesium But for most of the transition. The highest principal quantum number is 2. Calcium would have two valence electrons, since it is in group iia (group 2). Magnesium has two valence electrons. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level which is the.. Chlorine Valence Electrons In Magnesium.