What Is Considered A Medical Device . Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Definition of a medical device. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Either class i, ii or iii,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a.
from www.qmswrapper.com
Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Definition of a medical device. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a.
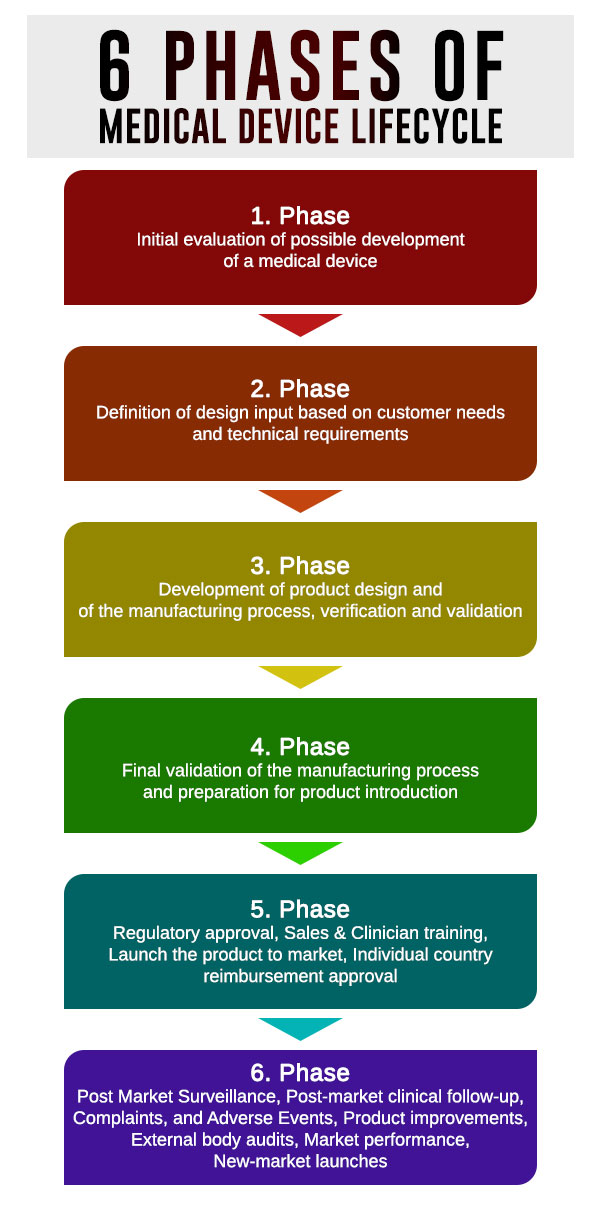
What is medical devices lifecycle qmsWrapper
What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Either class i, ii or iii,. Definition of a medical device.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation What Is Considered A Medical Device The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as:. What Is Considered A Medical Device.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from the leading EMS medical What Is Considered A Medical Device Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. A medical device is considered any apparatus that does not exert its action through chemical means and is. What Is Considered A Medical Device.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. The food and drug administration (fda) has established classifications for approximately 1,700 different. What Is Considered A Medical Device.
From www.massdevice.com
An expert's guide to developing medical devices MassDevice What Is Considered A Medical Device Definition of a medical device. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. A medical device is considered any apparatus that. What Is Considered A Medical Device.
From www.presentationeze.com
FDA medical device classification PresentationEZE What Is Considered A Medical Device Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Either class. What Is Considered A Medical Device.
From www.qmswrapper.com
What is medical devices lifecycle qmsWrapper What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Any medical device approved by the fda center for devices and radiological health is classified into one. What Is Considered A Medical Device.
From devtechnosys.com
What Is Medical Device And How Mobile App Can Be A Medical Device? What Is Considered A Medical Device The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Definition of a medical device. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. A. What Is Considered A Medical Device.
From laegemiddelstyrelsen.dk
Development of medical devices What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Either class i, ii or iii,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and.. What Is Considered A Medical Device.
From www.qstockinventory.com
FDA and UDI Compliance for Medical Device Distribution What Is Considered A Medical Device Definition of a medical device. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Either class i, ii or iii,. A medical device is considered any apparatus that does not exert its action through chemical means and is. What Is Considered A Medical Device.
From healthcarebusinessclub.com
All about the Pharmaceutical and Medical Device Industry Infographic Healthcare Business Club What Is Considered A Medical Device Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The food. What Is Considered A Medical Device.
From www.arenasolutions.com
Medical Device Definition Arena What Is Considered A Medical Device Definition of a medical device. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Either class i, ii or iii,. A medical device is considered any apparatus that does not exert its action through chemical means and is. What Is Considered A Medical Device.
From laegemiddelstyrelsen.dk
Medical devices What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Definition of a medical device. Section 201(h) of the food, drug & cosmetic. What Is Considered A Medical Device.
From starfishmedical.com
5 Tips for medical device cyber security What Is Considered A Medical Device Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Definition of a medical device. A medical device is considered any apparatus that does not exert its action through chemical means and is used. What Is Considered A Medical Device.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group What Is Considered A Medical Device Definition of a medical device. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: A medical device is considered any apparatus that does not exert its action through chemical means and is used in. What Is Considered A Medical Device.
From vem-medical.com
Medical Device Manufacturing What Is Considered A Medical Device A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Either class i, ii or iii,. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to.. What Is Considered A Medical Device.
From www.corpseed.com
Classification of Medical Devices by CDSCO in India Corpseed What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Definition of a medical device. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines. What Is Considered A Medical Device.
From www.researchgate.net
Medical wearables devices a) wearable electronics come into contact... Download Scientific What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Definition of a medical device. A medical. What Is Considered A Medical Device.
From www.pinterest.fr
EU Medical Device Classification Form Easy Medical Device What Is Considered A Medical Device A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Either class i, ii or iii,.. What Is Considered A Medical Device.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations MedicalDevices FDA What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Definition of a medical device. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. A medical device is considered any apparatus that. What Is Considered A Medical Device.
From www.raondigital.com
Connected Medical Devices Have Made The Lives Of Medical Providers And Patients Much Easier What Is Considered A Medical Device Either class i, ii or iii,. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease.. What Is Considered A Medical Device.
From angelanjohnson.com
Medical Devices Angela N Johnson What Is Considered A Medical Device Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Either class. What Is Considered A Medical Device.
From proqc.com
Manufacturing Medical Devices COVID 19 What Is Considered A Medical Device Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii,. A medical device is considered any apparatus that does not exert its action through chemical means and is used. What Is Considered A Medical Device.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Latest Standards YouTube What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii,. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Definition of a medical device. The food. What Is Considered A Medical Device.
From medium.com
What is the Role of Portable Medical Devices in Modern Healthcare? by Clinmedhealthcare Medium What Is Considered A Medical Device The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as. What Is Considered A Medical Device.
From thehealthcareinsights.com
Essential medical devices that should be available at your home The Healthcare Insights What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Any medical device approved by the fda center for devices and radiological health is classified into one of. What Is Considered A Medical Device.
From www.youtube.com
What are medical devices? YouTube What Is Considered A Medical Device Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention. What Is Considered A Medical Device.
From woodleybioreg.com
Medical devices what are they, and how do we know that they're safe? Woodley BioReg What Is Considered A Medical Device The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Any medical device approved by the fda center for devices and radiological health is classified. What Is Considered A Medical Device.
From amb.se
10 standards for medical devices AMB What Is Considered A Medical Device Either class i, ii or iii,. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll. What Is Considered A Medical Device.
From www.sciencetimes.com
How We Use Smart Devices To Monitor Our Health Science Times What Is Considered A Medical Device Either class i, ii or iii,. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: A medical device is considered any apparatus that does not exert its action through chemical means and is. What Is Considered A Medical Device.
From www.medicaldesignandoutsourcing.com
How to name a medical device Medical Design and Outsourcing What Is Considered A Medical Device Either class i, ii or iii,. Definition of a medical device. Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The food and drug administration (fda) has established classifications for approximately 1,700 different. What Is Considered A Medical Device.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE What Is Considered A Medical Device Definition of a medical device. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. The. What Is Considered A Medical Device.
From synectic.net
Medical Device FDA Regulations Infographic Synectic What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. Either class i, ii or iii,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Any medical device approved by the fda center for devices and radiological. What Is Considered A Medical Device.
From imaginationeering.com
Stages Involved in Designing of A Medical Product Device What Is Considered A Medical Device Medical devices in this class include defibrillators, pacemakers, breast implants, and some diagnostic software as a. Definition of a medical device. A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation, therapy, or prevention of disease. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines. What Is Considered A Medical Device.
From www.conveyco.com
Medical Device & Pharmaceutical Products What Is Considered A Medical Device The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: A medical device is considered any apparatus that does not exert its action through chemical means and is used in diagnosis, mitigation,. What Is Considered A Medical Device.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc What Is Considered A Medical Device Learn how to determine whether the product you're working on is considered a medical device and the necessary quality and regulatory steps you'll need to take to. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device is considered any apparatus that does not exert its action through chemical. What Is Considered A Medical Device.