Tin Iodate Formula . tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. iodimetric titration of tin ( sn(ii) ) sample preparation: you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. This yields a result of. to write the formula for tin (ii) iodide we’ll use the periodic table, a. Hydrochloric acid and 90 ml deionized water. 1.) add 50 ml conc. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Determine the amount of tin using moles = mass/molar mass. We will start with binary ionic,. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. write an equation for this reaction. An older system of nomenclature for such. It was first obtained in 2020 through the hydrothermal. It has a formula weight of 372.519.
from www.t3db.ca
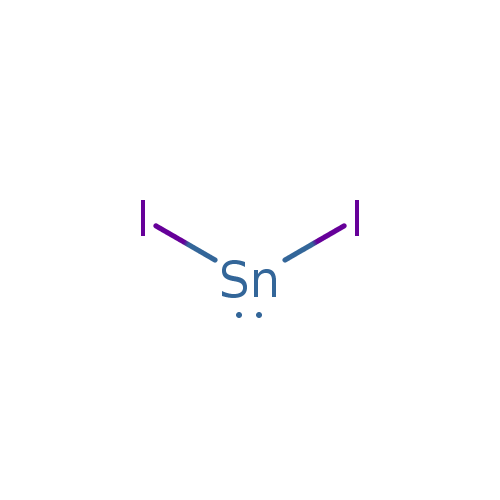
This yields a result of. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. iodimetric titration of tin ( sn(ii) ) sample preparation: to write the formula for tin (ii) iodide we’ll use the periodic table, a. It has a formula weight of 372.519. Hydrochloric acid and 90 ml deionized water. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. write an equation for this reaction. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).
T3DB Tin(II) iodide
Tin Iodate Formula This yields a result of. Hydrochloric acid and 90 ml deionized water. Determine the amount of tin using moles = mass/molar mass. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). to write the formula for tin (ii) iodide we’ll use the periodic table, a. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. iodimetric titration of tin ( sn(ii) ) sample preparation: We will start with binary ionic,. write an equation for this reaction. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. It has a formula weight of 372.519. This yields a result of. 1.) add 50 ml conc. An older system of nomenclature for such. It was first obtained in 2020 through the hydrothermal.
From www.numerade.com
SOLVED Complete the following table Some polyatomic ions name Tin Iodate Formula We will start with binary ionic,. This yields a result of. to write the formula for tin (ii) iodide we’ll use the periodic table, a. write an equation for this reaction. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. iodimetric titration of tin. Tin Iodate Formula.
From www.t3db.ca
T3DB Tin(IV) iodide Tin Iodate Formula to write the formula for tin (ii) iodide we’ll use the periodic table, a. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. It was first obtained in 2020 through the hydrothermal. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4.. Tin Iodate Formula.
From www.dreamstime.com
3D Image of Potassium Iodate Skeletal Formula Stock Illustration Tin Iodate Formula We will start with binary ionic,. It was first obtained in 2020 through the hydrothermal. iodimetric titration of tin ( sn(ii) ) sample preparation: Determine the amount of tin using moles = mass/molar mass. This yields a result of. An older system of nomenclature for such. It has a formula weight of 372.519. 1.) add 50 ml conc. . Tin Iodate Formula.
From www.youtube.com
Briefing Video Tin Iodide YouTube Tin Iodate Formula tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. to write the formula for tin (ii) iodide we’ll use the periodic table, a. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. 1.) add 50. Tin Iodate Formula.
From www.fishersci.com
Alfa Aesar Tin(IV) iodide, ultra dry, 99.998 (metals basis) Fisher Tin Iodate Formula This yields a result of. to write the formula for tin (ii) iodide we’ll use the periodic table, a. It has a formula weight of 372.519. iodimetric titration of tin ( sn(ii) ) sample preparation: write an equation for this reaction. We will start with binary ionic,. Determine the amount of tin using moles = mass/molar mass.. Tin Iodate Formula.
From www.youtube.com
How to Write the Formula for Tin (II) iodide YouTube Tin Iodate Formula tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. iodimetric titration of tin ( sn(ii) ) sample preparation: 1.) add 50 ml conc. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of. Hydrochloric acid and 90 ml deionized. Tin Iodate Formula.
From www.pw.live
Ammonium Iodide Formula Structure, Chemical Formula And Properties Tin Iodate Formula This yields a result of. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. to write the formula for tin (ii) iodide we’ll use the periodic table, a. Determine the amount of tin using moles = mass/molar mass. Hydrochloric acid and 90 ml deionized water. . Tin Iodate Formula.
From www.shutterstock.com
Potassium Iodate Formula Handwritten Chemical Formula Stock Tin Iodate Formula iodimetric titration of tin ( sn(ii) ) sample preparation: Determine the amount of tin using moles = mass/molar mass. This yields a result of. We will start with binary ionic,. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. Hydrochloric acid and 90 ml deionized water. It was first obtained in 2020 through the. Tin Iodate Formula.
From www.youtube.com
IO3 Lewis Structure How to Draw the Lewis Structure for the Iodate Tin Iodate Formula to write the formula for tin (ii) iodide we’ll use the periodic table, a. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). iodimetric titration of tin ( sn(ii) ) sample preparation: 1.) add 50 ml conc. It was first. Tin Iodate Formula.
From testbook.com
Ammonium Iodide Formula Explained with Preparation and its Uses. Tin Iodate Formula write an equation for this reaction. This yields a result of. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). We. Tin Iodate Formula.
From fr.slideshare.net
Rappels de nomenclature en chimie p12 Tin Iodate Formula write an equation for this reaction. Determine the amount of tin using moles = mass/molar mass. It has a formula weight of 372.519. We will start with binary ionic,. It was first obtained in 2020 through the hydrothermal. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. This yields a result of. you. Tin Iodate Formula.
From www.pngegg.com
Tin(IV) chloride Tin(IV) iodide Tin(II) chloride Molecule, Tin, purple Tin Iodate Formula It has a formula weight of 372.519. Determine the amount of tin using moles = mass/molar mass. We will start with binary ionic,. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. 1.) add 50 ml conc. tin(iv) iodate is an inorganic compound with the chemical. Tin Iodate Formula.
From www.adda247.com
Potassium Iodate Formula (KIO3) Structure, Properties, Uses Tin Iodate Formula This yields a result of. Determine the amount of tin using moles = mass/molar mass. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). It has a formula weight of 372.519. An older system of nomenclature for such. 1.) add 50 ml. Tin Iodate Formula.
From www.pw.live
Potassium Iodate Formula, Structure, Properties, Uses Tin Iodate Formula write an equation for this reaction. An older system of nomenclature for such. It was first obtained in 2020 through the hydrothermal. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. to write the formula for tin (ii) iodide we’ll use the periodic table, a. We will start with binary ionic,. 1.) add. Tin Iodate Formula.
From www.t3db.ca
T3DB Tin(II) iodide Tin Iodate Formula write an equation for this reaction. An older system of nomenclature for such. It was first obtained in 2020 through the hydrothermal. This yields a result of. We will start with binary ionic,. to write the formula for tin (ii) iodide we’ll use the periodic table, a. Determine the amount of tin using moles = mass/molar mass. 1.). Tin Iodate Formula.
From www.smart-elements.com
Tin(IV) iodide large tin tetra iodide crystals 99.99 Tin Iodate Formula tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrochloric acid and 90 ml deionized water. This yields a result of. . Tin Iodate Formula.
From www.chegg.com
Solved Experiment 4. Tin lodide Synthesis Short Overview. Tin Iodate Formula An older system of nomenclature for such. Hydrochloric acid and 90 ml deionized water. 1.) add 50 ml conc. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. It was first obtained in 2020 through the hydrothermal. We will start with binary ionic,. It has a formula weight of. Tin Iodate Formula.
From www.fishersci.ie
Tin(II) iodide, 99+, Thermo Scientific Chemicals Fisher Scientific Tin Iodate Formula We will start with binary ionic,. An older system of nomenclature for such. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). This yields a result of. Determine the amount of tin using moles = mass/molar mass. you then figure the. Tin Iodate Formula.
From www.youtube.com
How to write the formula for potassium iodate YouTube Tin Iodate Formula you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. 1.) add 50 ml conc. Determine the amount of tin using moles = mass/molar mass. This yields a result of. It was first obtained. Tin Iodate Formula.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download Tin Iodate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such. Hydrochloric acid and 90 ml deionized water. Determine the amount of tin using moles = mass/molar mass. It has a formula weight of 372.519. tin(iv). Tin Iodate Formula.
From www.fishersci.com
Sodium Iodate (Powder/Certified), Fisher Chemical, Quantity 100 g Tin Iodate Formula 1.) add 50 ml conc. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. An older system of nomenclature for such. iodimetric titration of tin ( sn(ii) ) sample preparation: Determine the amount of tin using moles = mass/molar mass. Hydrochloric acid and 90 ml deionized. Tin Iodate Formula.
From www.anyrgb.com
Tin Bromide, tiniv Iodide, tinii Fluoride, tiniv Chloride, tiniv Tin Iodate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrochloric acid and 90 ml deionized water. write an equation for this reaction. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. This yields a result of.. Tin Iodate Formula.
From www.numerade.com
SOLVED Which of the following would be the reaction at the anode in Tin Iodate Formula tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. It was first obtained in 2020 through the hydrothermal. It has a formula weight of 372.519. An older system of nomenclature for such. to write the formula for tin (ii) iodide we’ll use the periodic table, a. thus cu + is copper(i) (read as. Tin Iodate Formula.
From bakerpedia.com
Sodium Iodate Baking Ingredients BAKERpedia Tin Iodate Formula iodimetric titration of tin ( sn(ii) ) sample preparation: tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of. An older system of nomenclature for such. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. We will start with. Tin Iodate Formula.
From www.alamy.com
3D image of Calcium iodate skeletal formula molecular chemical Tin Iodate Formula It was first obtained in 2020 through the hydrothermal. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. iodimetric titration of tin ( sn(ii) ) sample preparation:. Tin Iodate Formula.
From slideplayer.com
HC CHEMISTRY HC CHEMISTRY (B) Periodicity Bonding Continuum. ppt download Tin Iodate Formula An older system of nomenclature for such. It was first obtained in 2020 through the hydrothermal. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. This yields a result of. write an equation for this reaction. you then figure the formula based on the lowest whole number. Tin Iodate Formula.
From www.pinpng.com
Tin Iodide 2d Dimensions Tin Molecule, HD Png Download 1100x1020 Tin Iodate Formula write an equation for this reaction. iodimetric titration of tin ( sn(ii) ) sample preparation: tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. An older system of nomenclature for such. 1.) add 50 ml conc. you then figure the formula based on the lowest whole. Tin Iodate Formula.
From encyclopedia.pub
Tiniodate rechargeable battery Encyclopedia MDPI Tin Iodate Formula It was first obtained in 2020 through the hydrothermal. It has a formula weight of 372.519. This yields a result of. iodimetric titration of tin ( sn(ii) ) sample preparation: to write the formula for tin (ii) iodide we’ll use the periodic table, a. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4.. Tin Iodate Formula.
From www.youtube.com
How to write chemical formula Tin IV Oxide YouTube Tin Iodate Formula Determine the amount of tin using moles = mass/molar mass. iodimetric titration of tin ( sn(ii) ) sample preparation: tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. It has a formula weight of 372.519. to write the formula for tin (ii) iodide we’ll use the periodic. Tin Iodate Formula.
From www.numerade.com
SOLVED Iron(II) iodate Express your answer as a chemical formula. Tin Iodate Formula tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. 1.) add 50 ml conc. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is. Tin Iodate Formula.
From www.slideserve.com
PPT Name the Chemical Compound PowerPoint Presentation, free download Tin Iodate Formula tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. Hydrochloric acid and 90 ml deionized water. iodimetric titration of tin ( sn(ii) ) sample preparation: It was first obtained in 2020 through the hydrothermal. This yields a result of. We will start with binary ionic,. It has a formula weight of 372.519. you. Tin Iodate Formula.
From www.youtube.com
Hown to make tin(IV) iodide YouTube Tin Iodate Formula iodimetric titration of tin ( sn(ii) ) sample preparation: tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. It was first obtained in 2020 through the hydrothermal. tin(iv) iodate is an inorganic compound with the chemical formula sn(io 3) 4. Determine the amount of tin using moles. Tin Iodate Formula.
From pubs.acs.org
Crystal Structure Evolution and Notable Thermal Expansion in Hybrid Tin Iodate Formula tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. It was first obtained in 2020 through the hydrothermal. Hydrochloric acid and 90 ml deionized water. you then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. to. Tin Iodate Formula.
From molekula.com
Purchase Sodium iodate [7681552] online • Catalog • Molekula Group Tin Iodate Formula Determine the amount of tin using moles = mass/molar mass. It was first obtained in 2020 through the hydrothermal. iodimetric titration of tin ( sn(ii) ) sample preparation: It has a formula weight of 372.519. write an equation for this reaction. An older system of nomenclature for such. Hydrochloric acid and 90 ml deionized water. to write. Tin Iodate Formula.
From www.youtube.com
Sodium iodate YouTube Tin Iodate Formula This yields a result of. tin(ii) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula sni 2. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). We will start with binary ionic,. you. Tin Iodate Formula.